Method for detecting related substances in chloroquine phosphate tablet
A technology of chloroquine phosphate tablets and detection methods, applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as difficult to obtain, relative retention time, relative response factor confirmation, and difficult to accurately evaluate drug quality, so as to avoid purchasing Expensive, good resolution, effective separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The detection method of related substance in embodiment 1 chloroquine phosphate tablet
[0055] 1. Chromatographic conditions: fill the chromatographic column with octadecylsilane bonded silica gel (the length of the chromatographic column is 150mm or 250mm, the inner diameter of the chromatographic column is 4.6μm, and the particle size of the chromatographic filler is 5μm); connect the ultraviolet detector in series The CAD detector is a detector, the ultraviolet detection wavelength is 260nm, the atomization temperature of the CAD detector is 35°C, and the output range is 100pA; with 0.1% (v / v) trifluoroacetic acid aqueous solution: acetonitrile (volume ratio) = 35:65 It is the mobile phase; the flow rate is 1.0ml / min; the column temperature is 30°C; the injection volume is 20μl.
[0056] The experimental steps are as follows:
[0057] (1) Preparation of the test solution: get the chloroquine phosphate tablet and grind it finely, accurately weigh an appropriate amou...
Embodiment 2
[0081] Embodiment 2: methodological investigation
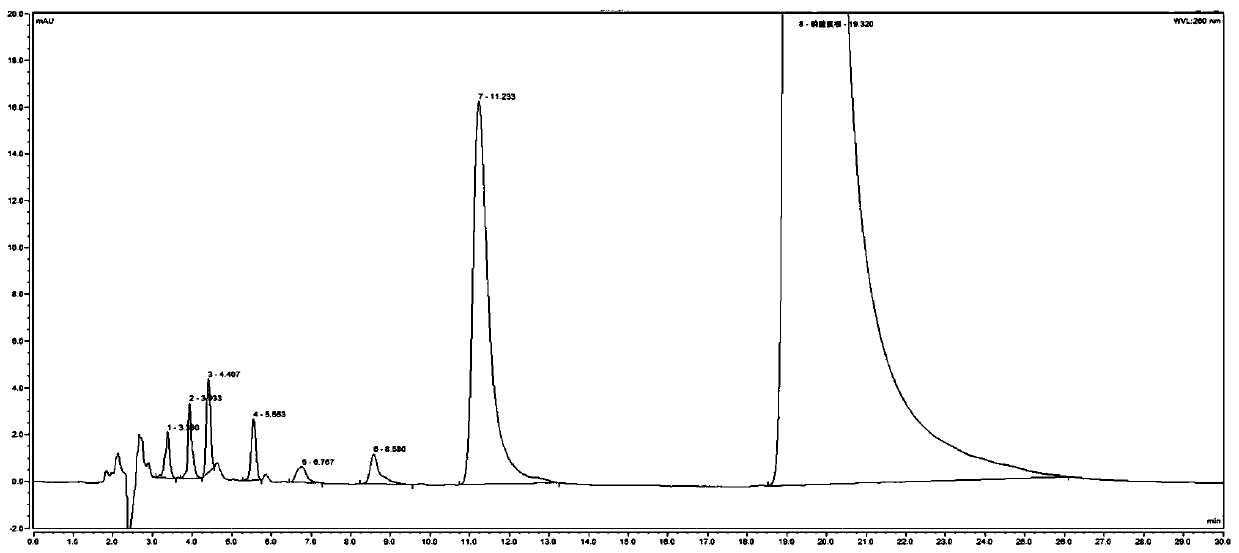
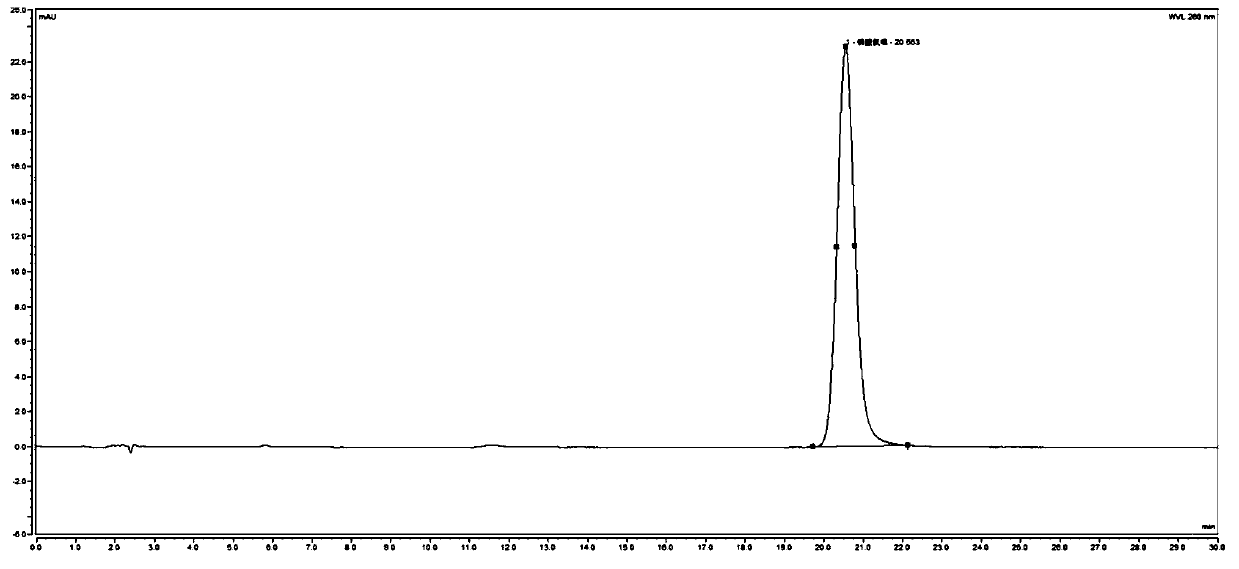
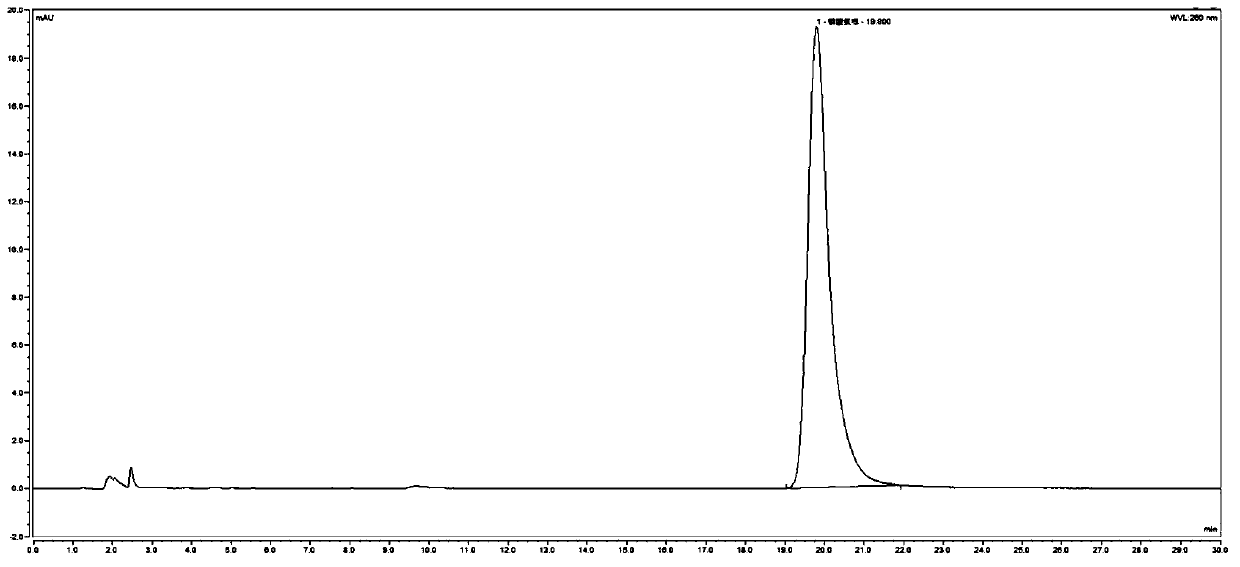
[0082] 1. System adaptability inspection:
[0083] (1) Preparation of the test solution: get TF0006 batches of chloroquine phosphate tablets and grind finely, accurately weigh an appropriate amount (approximately equivalent to chloroquine phosphate 0.10g), put in a 50ml measuring bottle, dilute with mobile phase, ultrasonically dissolve, and settle to volume scale, shake well, filter, and get the continued filtrate to obtain the test solution;
[0084] (2) Preparation of system adaptability solution: take the above-mentioned test solution, irradiate it with an ultraviolet lamp at 365nm for 24 hours, pass through an organic filter membrane with a pore size of 0.45 μm, and obtain it;
[0085] (3) Preparation of contrast solution: precisely measure 1 ml of the test solution and put it in a 100 ml measuring bottle, add mobile phase to constant volume, shake well, and get final product;
[0086] (4) Reference substance solution:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com