Application of umbilical cord mesenchymal stem cells in preparation of medicine for treating novel coronal pneumonia
A technology of mesenchymal stem cells and umbilical cord, which is applied in the application field of umbilical cord mesenchymal stem cells in the preparation of drugs for the treatment of new coronary pneumonia, can solve problems such as the incomplete understanding of the pathogenesis, and achieve the relief of acute respiratory distress syndrome, pulmonary edema, The effect of reducing the fatality rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Before the application of hUMSCs in clinic, the inventors of the present application found that hUMSCs cell culture medium has good immune regulation function.
[0025] Materials and methods:
[0026] Animals: Male C57BL / 6 mice.
[0027] Cells: human umbilical cord mesenchymal stem cells (hUC-MSCs).
[0028] Cell processing method: press 1×10 6 Density The culture solution of hUMSCs cells cultured in a 10 cm dish for 48 hours was filtered through a 0.22 μm filter membrane (Millipore) to remove cells or cell debris, and concentrated 100 times using an ultrafiltration centrifuge tube.
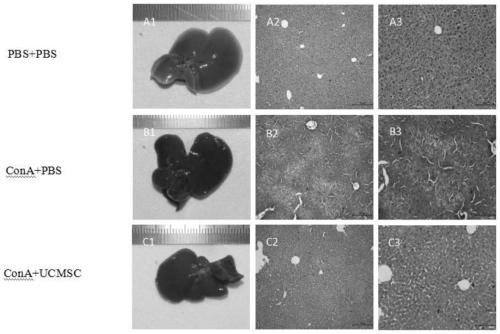
[0029] Acute immune liver injury model production method: 18 male C57BL / 6 mice were divided into 3 groups, the model control group (ConA+PBS), according to 25mg / kg body weight through the tail vein injection of concanavalin dissolved in PBS to induce acute liver injury. After injury, 30 minutes later, inject PBS through the tail vein; the negative control group (PBS+PBS), that is, use P...
Embodiment 2
[0036] hUMSCs after expansion and culture use physiological saline + 1% human serum albumin + 2‰ nadroparin calcium injection to make cell suspension pharmaceutical preparations, and 100mL cell suspension contains 5 × 10 7 mesenchymal stem cells.
[0037] In this embodiment, the cell suspension pharmaceutical preparation is used in critically ill covid19 patients with new coronary pneumonia, and the basic conditions of the patients are as follows:
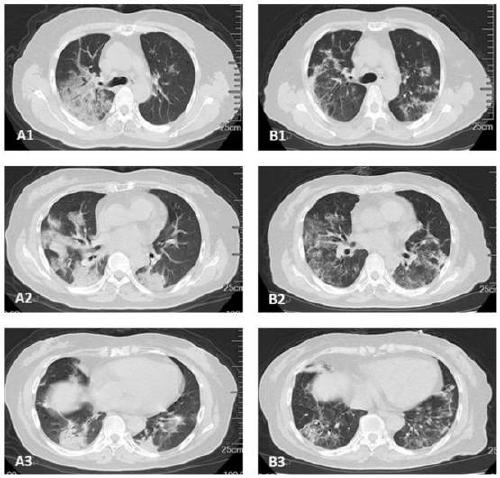
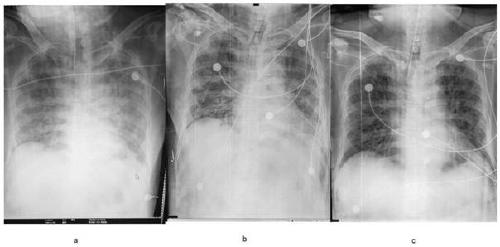
[0038] After administration, the patient's physical indicators such as Figure 3 ~ Figure 5 shown. Antibiotics are used to prevent infection during treatment, along with thymosin α1. After the first injection, no obvious side effects were observed and the drug was well tolerated. As shown in Table 1, after the second administration, serum bilirubin, CRP, and ALT / AST gradually decreased, and other vital signs improved. From February 13th, the endotracheal tube was also removed and the patient was able to walk on the ground. ...
Embodiment 3
[0049] Isolation and expansion of umbilical cord mesenchymal stem cells and preparation of cell products
[0050] Seed bank primary isolation
[0051] Confirm the donor’s information, medical history, and infectious disease conditions before entering the seed bank (the donor’s HBV, HCV, HIV, and syphilis tests were negative). The isolated umbilical cord has no edema, no folds, and is elastic. Umbilical cord mesenchymal stem cells were cultured to the P2 generation, and a seed bank was established. The cells were cultured with medium MSCPro XenoFree medium (brand: rFib; product number: CCM0016-01).
[0052] The specific operation steps are:
[0053] 1.1 Prepare tissue digestion solution, including collagenase, hyaluronidase, gentamicin sulfate and DEME / F-12;
[0054] 1.2 Take out the umbilical cord, discard the clean umbilical cord blood, separate Wharton’s jelly, and repeatedly cut into pieces of tissue of about 0.5-1mm3 in the collagenase solution for 3 minutes to 15 minut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com