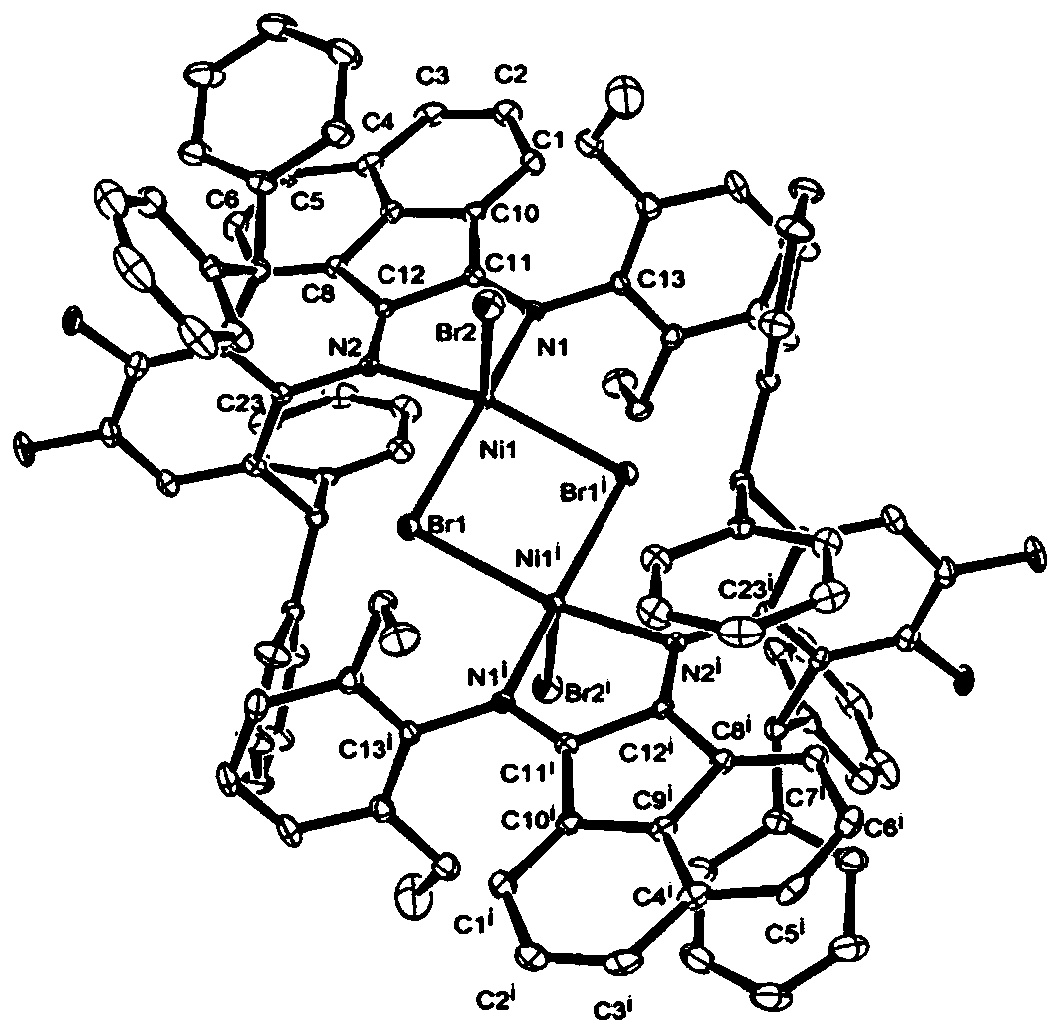
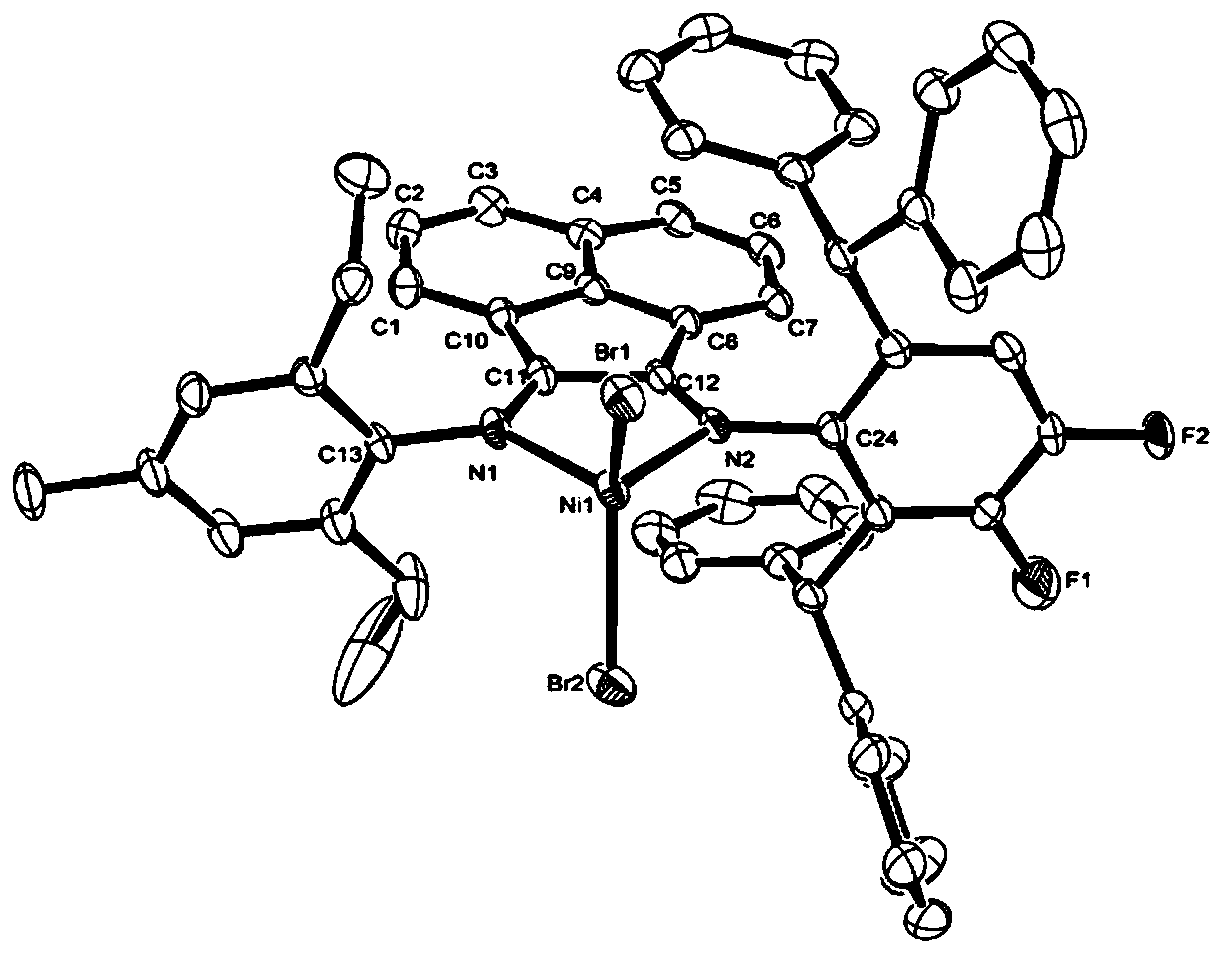
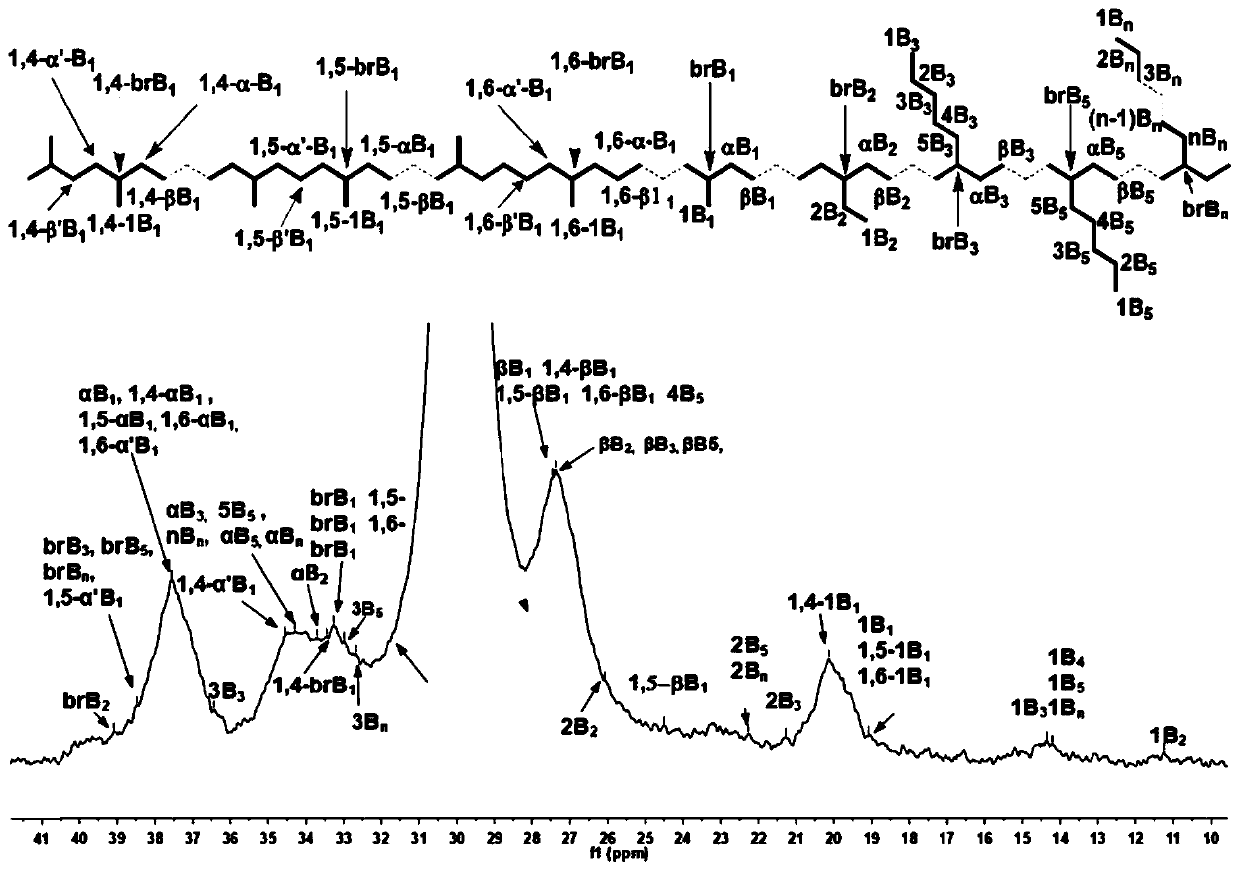
Fluorine-containing alpha-diimine nickel coordination compounds for preparing polyolefin elastomer, intermediate, preparation method and application thereof
A technology of polyolefin elastomer and diimine ligand, which is applied in the preparation of nickel organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, amino compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] In this example, 2-(2,6-bis(benzhydryl)-3,4-difluoroanilino)acenaphthylenone represented by formula (V) was prepared.
[0099] In 2,6-bis(benzhydryl)-3,4-difluoroaniline (4.61g, 10.0mmol) and acenaphthylenedione (1.82g, 10.0mmol) in dichloromethane (150mL) and ethanol (30mL) A catalytic amount (0.57 g) of p-toluenesulfonic acid was added to the mixed solution, and the mixture was reacted at room temperature for 12 h. The solvent was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 5:1, and the eluted fraction was detected through a thin-layer silica gel plate, and the developing solvent was petroleum ether and ethyl acetate. The volume ratio of the ester was 5:1 mixed solvent, the third fraction was collected, and a yellow solid was obtained after removing the solvent. Yield: 49%.
[0100] The structural confirmation data are as follows:
[0101] 1 H NMR (400M...
Embodiment 2
[0107] In this example, the α-diimine intermediate represented by formula (II): 1-(2,6-dimethylaniline)-2-(2,6-bis(benzhydryl)-3 , 4-difluoroaniline) acenaphthene [L1], where R 1 is methyl, R 2 for hydrogen.
[0108] Get 2-(2,6-bis(benzhydryl)-3,4-difluoroaniline) acenaphthenone (0.625g, 1.0mmol) and 2,6-dimethylaniline (0.111 g, 1.0mmol) in toluene solution (50mL) was added a catalytic amount of p-toluenesulfonic acid, heated to reflux for 12h. The solvent toluene was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 5:1. The eluted fractions were examined through a thin-layer silica gel plate, the third fraction was collected, and the solvent was removed to obtain a red solid. Yield: 17%.
[0109] The structural confirmation data are as follows:
[0110] 1 H NMR (400MHz, CDCl 3 ,TMS): δ7.69(d,J=11.2Hz,1H),7.49(d,J=10.8Hz,1H),7.28(s,1H),7.20(d,J=10.0Hz,3H),7.17 ...
Embodiment 3
[0116] In this example, the α-diimine intermediate represented by formula (II) was prepared: 1-(2,6-diethylaniline)-2-(2,6-bis(benzhydryl)-3 , 4-difluoroaniline) acenaphthene [L2], where R 1 is ethyl, R 2 for hydrogen.
[0117] Get 2-(2,6-bis(benzhydryl)-4-methylaniline) acenaphthylenone (0.625g, 1.0mmol) and 2,6-diethylaniline (0.149g, 1.0 mmol) of toluene (50 mL) was added a catalytic amount of p-toluenesulfonic acid, and heated to reflux for 12 h. The solvent toluene was removed, and the residue was subjected to basic alumina column chromatography with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 5:1. The eluted fractions were examined through a thin-layer silica gel plate, the third fraction was collected, and the solvent was removed to give a yellow solid. Yield: 13%.
[0118] The structural confirmation data are as follows:
[0119] 1 H NMR (400MHz, CDCl 3 ,TMS): δ7.65(d,J=11.2Hz,1H),7.45(d,J=11.2Hz,1H),7.27(s,2H),7.20(d,J=6.0Hz,8H),7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polymerization activity | aaaaa | aaaaa |
| Polymerization activity | aaaaa | aaaaa |
| Polymerization activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com