Monoclonal antibody for detecting tobacco apyrases and application of monoclonal antibody
An ATPase and tissue ATPase technology, which is applied in the field of monoclonal antibodies for detecting tobacco ATPase, can solve problems such as lack of antibody tools, and achieve the effect of high specificity and high affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of a monoclonal antibody to adenosine triphosphatase hybridoma cells and preparation and purification of an anti-adenosine triphosphatase monoclonal antibody
[0031] 1.1 Antigen preparation
[0032] Prokaryotic expression of whole adenosine triphosphatase protein as an immunogen, total protein-coupled VLPs, and immunogenicity enhancers of conventional KLH systems.
[0033] 1.2 Immunization of mice
[0034] Six Balb / c mice (8-12 weeks old) were immunized with the antigen, and their serum titers were monitored to determine the optimal number of immunizations. Optimized adjuvants and immunization methods can produce high-affinity antibodies (IgG subtypes) against most antigenic polypeptides. After the initial immunization, there will be 3 to 4 times of boosting. After the boosting, the mouse serum will be taken to detect the titer (the recombinant protein of adenosine triphosphatase is used as an anti-antigen coating). Mice with qualified titers ...
Embodiment 2
[0043] Example 2: Verification of Anti-ATPase Monoclonal Antibody
[0044] The obtained monoclonal antibody cell lines were verified by enzyme-linked immunosorbent immunoassay, western blotting, co-immunoprecipitation plus mass spectrometry, antibody chip, etc. to determine the effectiveness of the antibody.
[0045] 2.1 ELISA (enzyme-linked immunosorbent) verification of antibodies and antigenic peptides
[0046] Coat the 96-well ELISA plate with ascitic fluid antibody to be paired, incubate, wash and block overnight with skim milk, wash with PBS, and store at 4°C until use. Antigen peptides were incubated, washed with PBS, and controls were set at the same time. HRP-labeled detection antibody was added to the previously incubated ELISA plate. TMB color reaction, microplate reader reading. Antibody titers are shown in Table 1:
[0047] Table 1 Antibody ELISA detection data
[0048]
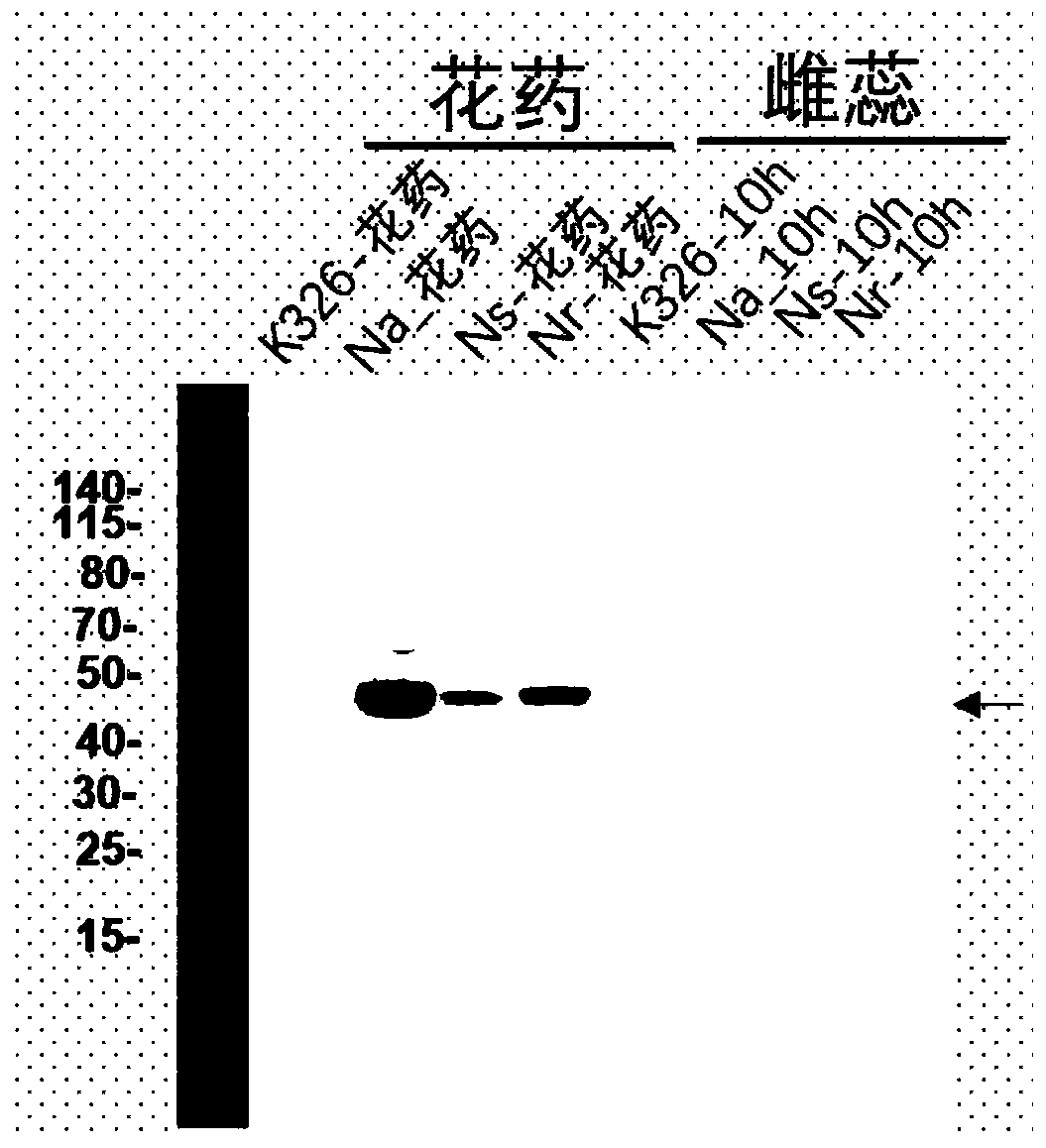
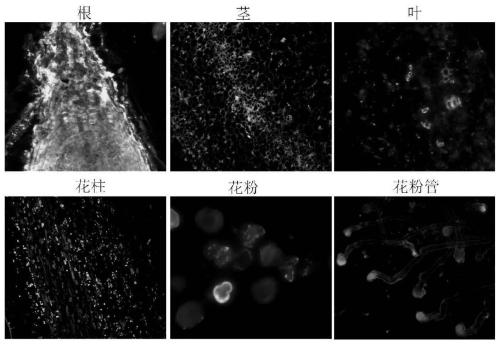
[0049] 2.2 Endogenous protein immunoblotting (WB) validation of antibodies
[0050] Us...
Embodiment 3
[0054] Example 3: Sequencing of Anti-ATPase Monoclonal Antibodies
[0055] The hybridoma cell line of anti-APY antibody was cultured and the total RNA was extracted, and the mRNA was reverse transcribed into the first-strand cDNA; the heavy chain and light chain genes were amplified by PCR and the amplified genes were cloned into the sequencing vector for multiple The final sequence results were obtained by sequencing the positive clones.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com