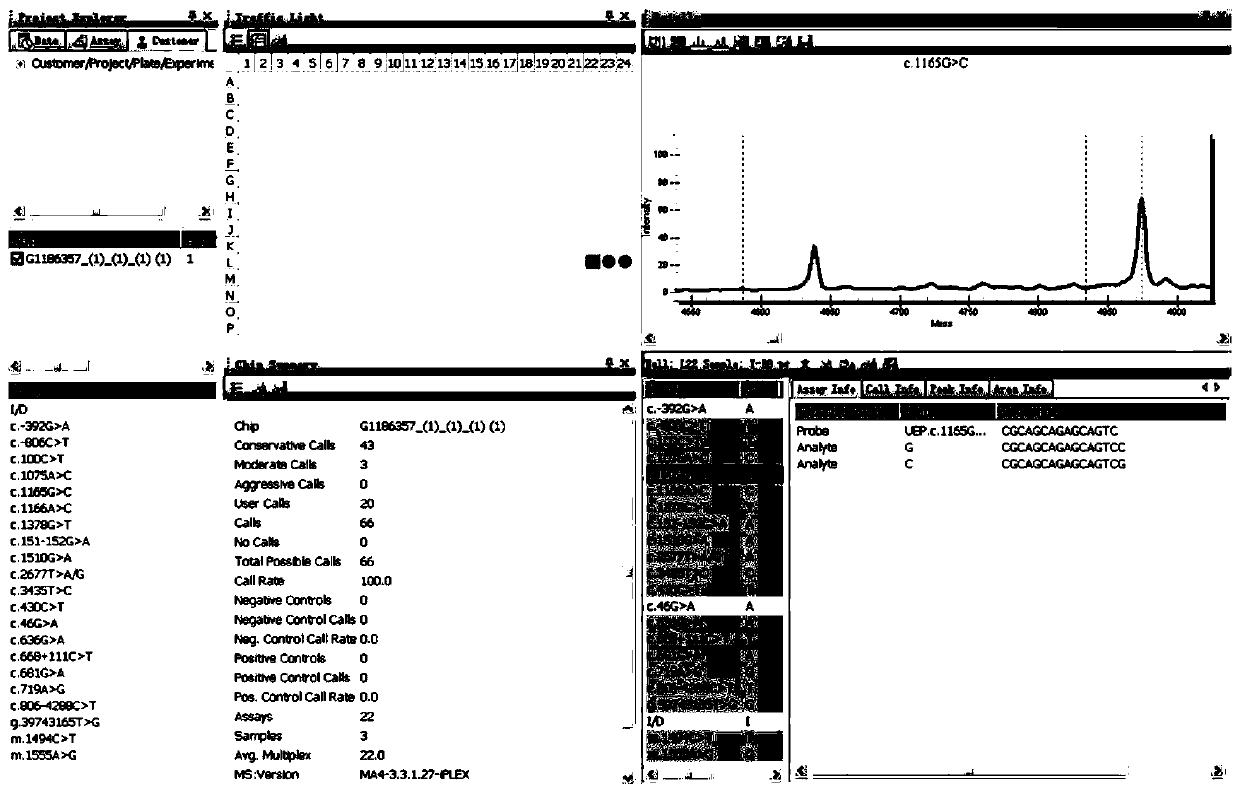
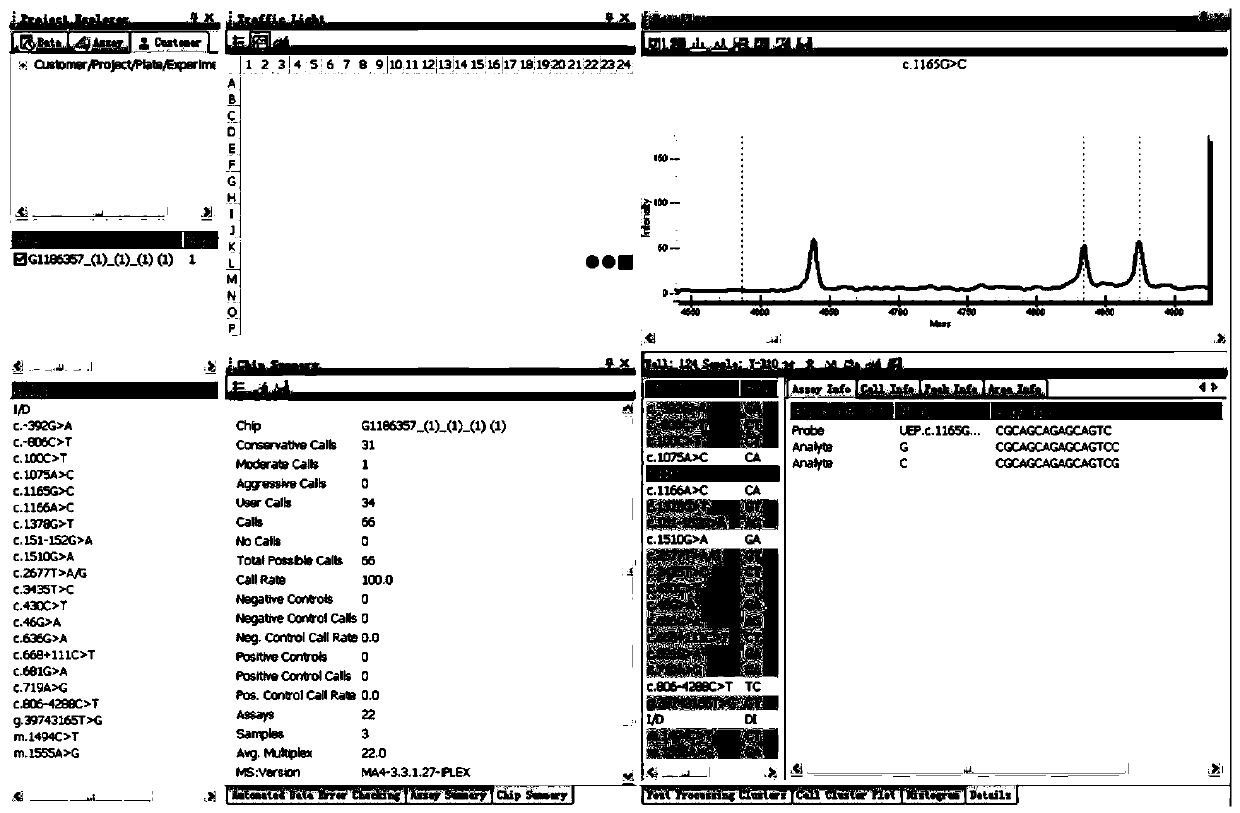
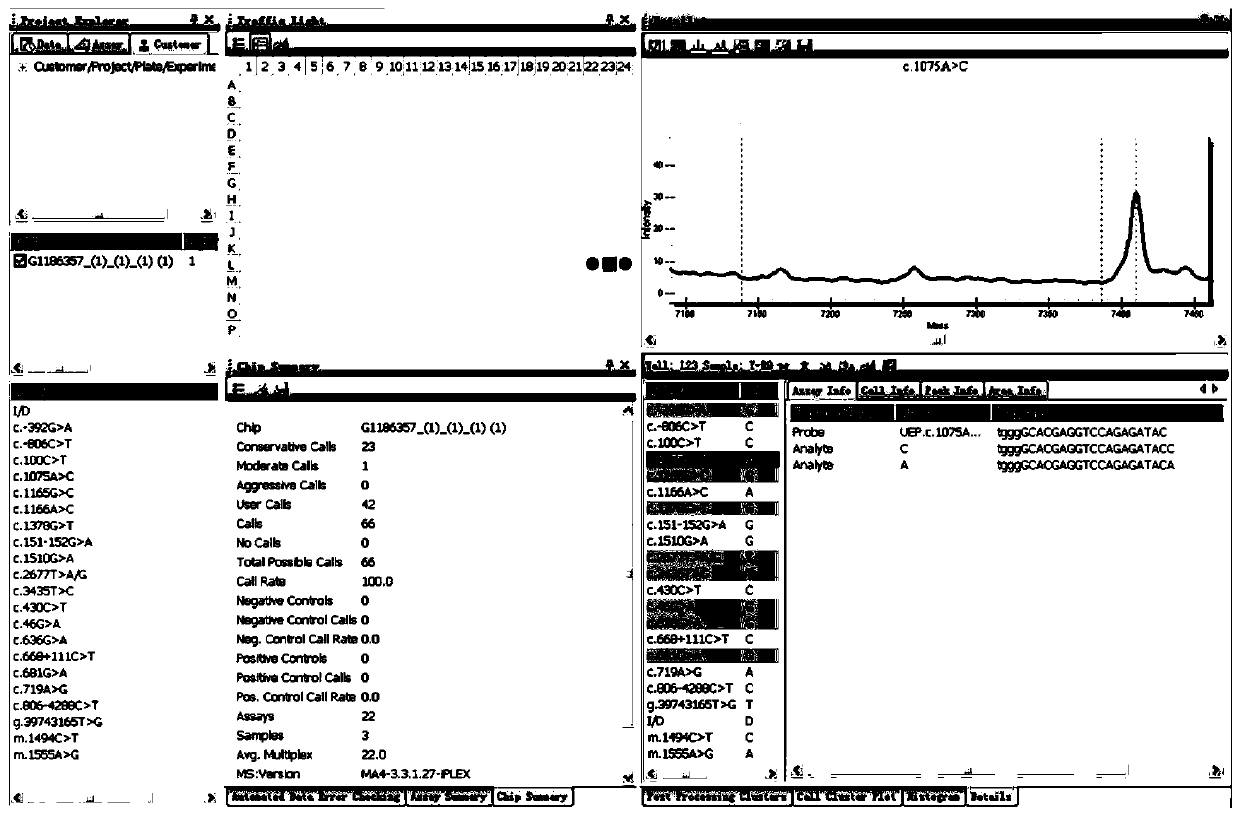
Primer sequence and test kit for gene detection of safe medication for children
A child-safe, genetic testing technology, applied in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., can solve the problems of difficult interpretation of results, high nucleic acid sample size, and easy non-specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: The primers for detecting children’s drug safety genes were synthesized by Shanghai Bailige Biotechnology Co., Ltd., and the sequence is as follows:
[0042] Amplification primers:
[0043]
[0044]
[0045] Single base extension primer:
[0046]
[0047]
Embodiment 2
[0048] Example 2: Detection of mutant plasmids at each site of the child safety drug gene was synthesized by Shanghai Sangon Bioengineering Co., Ltd.
Embodiment 3
[0049] Example 3: Preparation of a genetic detection kit for children's safe drug use.
[0050] (1) Nucleic acid sample pretreatment reagent for time-of-flight mass spectrometry detection system (Dipu Diagnostics, article number: 20020100), including the following main components:
[0051]
[0052] (2) Amplification reaction primer premix: a mixture of nucleotide sequences shown in SEQ ID NO: 1 to 45 in claim 1; the preferred solution is that the primers shown in SEQ ID NO: 1 to 44 The concentration is 0.5 μM, and the concentration of the primer shown in SEQ ID NO: 45 is 1.5 μM;
[0053] (3) Single-base extension reaction primer premix: a mixture of nucleotide sequences shown in SEQ ID NO: 46-67 in claim 2, and the preferred molar concentration of each extension primer is as follows:
[0054]
[0055]
[0056] (4) Desalting resin: including cation exchange resin powder for removing salt ions from the extension reaction solution;
[0057] (5) Detection chip: a silico...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com