Cell nucleus fluorescent probe with high brightness and high light stability
A fluorescent probe, high-brightness technology, applied in the field of fluorescence imaging, can solve the problems of time-consuming and labor-intensive, and achieve the effect of high photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
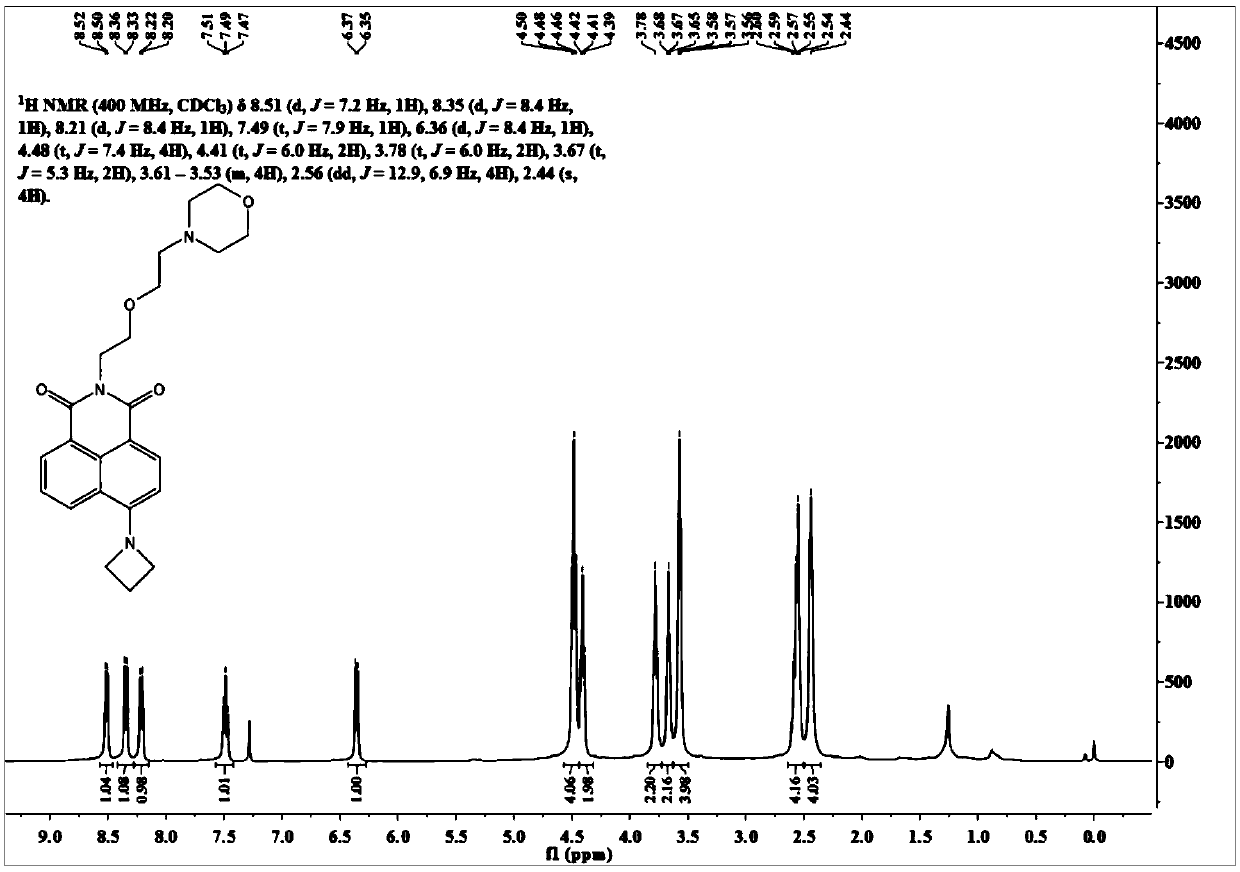
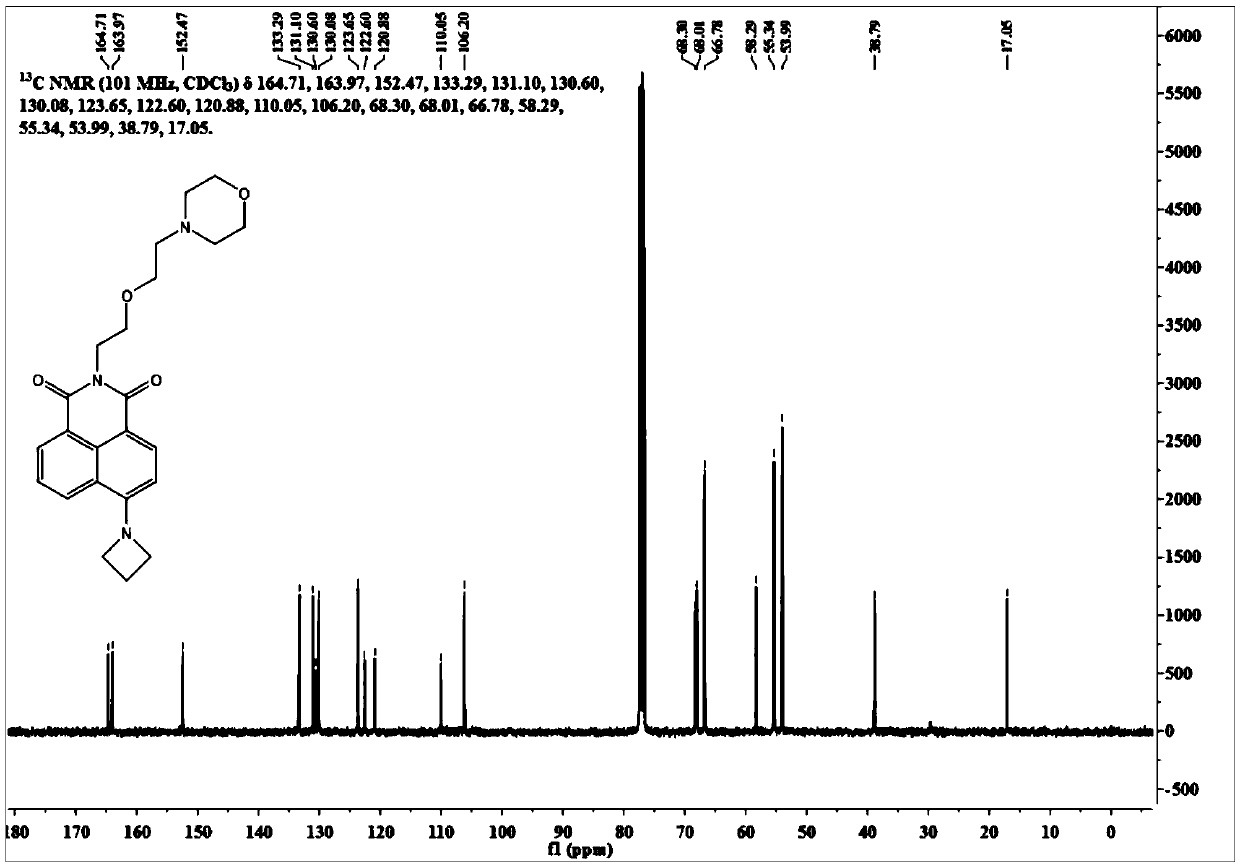
[0035] Synthesis of the nuclear probe OMor-Aze.
[0036] The intermediate Naph-Aze synthesis route and product structure are as follows:
[0037]
[0038] Weigh 4-bromo-1,8-naphthalene anhydride (3.0g, 10.9mmol), anhydrous copper sulfate (5.2g, 32.7mmol) in 10mL dry DMF, add azetidine (1.9mmol) under nitrogen protection g, 32.7 mmol), the temperature was raised to 140° C. to react for 20 h, and after cooling the reaction solution to room temperature, the reaction solution was slowly poured into ice water, and a large amount of orange solids were precipitated. The filtrate was removed by suction filtration, and the filter residue was dissolved in dichloromethane and purified by silica gel column chromatography (petroleum ether / dichloromethane=1 / 10, V / V) to obtain 1.1 g of orange solid powder Naph-Aze with a yield of 40%.
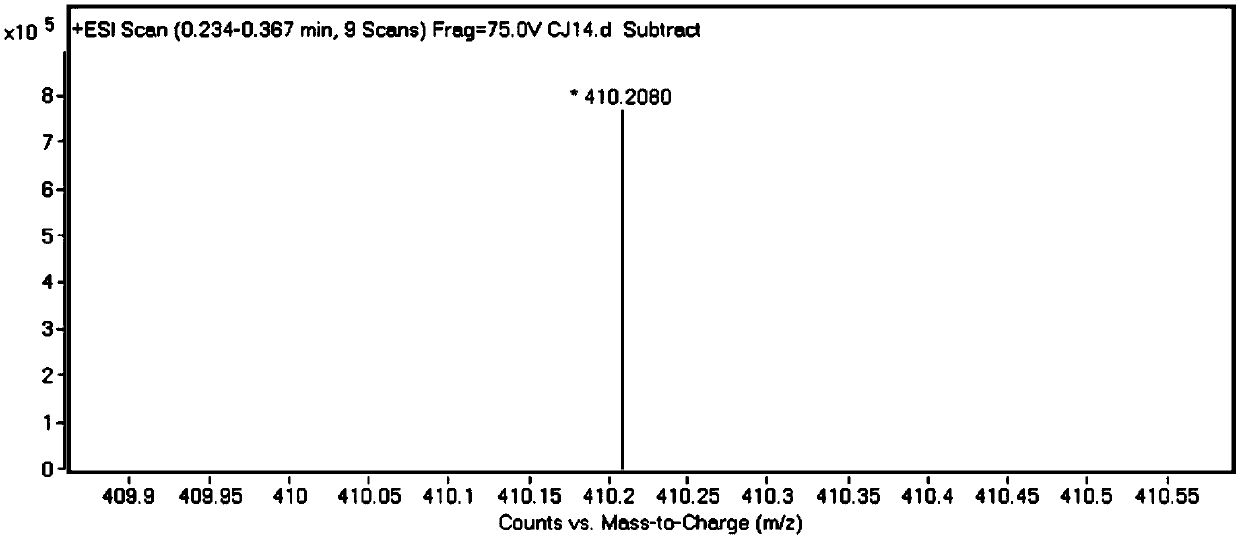
[0039] Its high-resolution mass spectrometry data are as follows:
[0040] HRMS (ESI): m / z: [M+H] + : Calculated: 254.0817, Experimented: 254.0883.
...
Embodiment 2
[0058] The synthesis route and product structure of nuclear probe Pid-Aze are as follows:
[0059]
[0060] Weigh Naph-Aze (0.1g, 0.4mmol) in 5mL of absolute ethanol, add 1-aminopiperidine (1.2g, 12mmol), heat the reaction solution to 80°C for 8h, then cool the reaction solution to room temperature, reduce The solvent was distilled off under high pressure, and the residue was separated through a silica gel column (dichloromethane / methanol=50 / 1, V / V) to obtain the product Pid-Aze as an orange solid 0.11 g with a yield of 87%.
[0061] Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0062] 1 H NMR (400MHz, CDCl 3 , ppm): δ=8.55(d, J=6.9Hz, 1H), 8.39(d, J=8.3Hz, 1H), 8.26(d, J=8.2Hz, 1H), 7.52(t, J=7.6Hz ,1H),6.41(d,J=8.3Hz,1H),4.51(t,J=6.9Hz,4H),3.12(t,J=7.2Hz,4H),2.61-2.52(m,2H),1.86 (m,4H),1.52(m,2H).
[0063] After testing, its structure is shown in the above formula Pid-Aze. Its ultraviolet absorption in ethanol is 450nm, and its fluorescence e...
Embodiment 3
[0065] The synthesis route and product structure of nuclear probe Pia-Aze are as follows:
[0066]
[0067] Weigh Naph-Aze (0.1g, 0.4mmol) in 5mL of absolute ethanol, add 1-(2-aminoethyl)piperazine (0.025g, 0.4mmol), heat the reaction solution to 80°C for 8h, The reaction solution was cooled to room temperature, and the solvent was distilled off under reduced pressure. The residue was separated through a silica gel column (dichloromethane / methanol=50 / 1, V / V) to obtain the product Pia-Aze as an orange solid 0.09 g, with a yield of 63%.
[0068] Its nuclear magnetic spectrum hydrogen spectrum data are as follows:
[0069] 1 H NMR (400MHz, CDCl 3 , ppm): δ=8.55(d, J=6.9Hz, 1H), 8.39(d, J=8.3Hz, 1H), 8.26(d, J=8.2Hz, 1H), 7.52(t, J=7.6Hz ,1H),6.41(d,J=8.3Hz,1H),4.51(t,J=6.9Hz,4H),4.32(d,J=6.1Hz,2H),3.70(s,4H),2.70(s ,2H),2.65-2.39(m,6H).
[0070] Its nuclear magnetic spectrum carbon spectrum data are as follows:
[0071] 13 C NMR (101MHz, CDCl 3 , ppm): δ=164.70, 163.98...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com