Coating method for improving ionic conductivity of positive electrode material of lithium ion battery and coated and modified positive electrode material
A technology for lithium-ion batteries and positive electrode materials, which is applied to battery electrodes, cylindrical shell batteries/batteries, positive electrodes, etc. It can solve the problems of affecting the ion conductivity of positive electrode materials and unspecific conduction of lithium ions, so as to suppress side reactions , Improve lithium ion conductivity, improve the effect of rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (1) Weigh 0.1g LiTFSI and 0.05g photoinitiator 184 and add to 0.895g polyethylene glycol diacrylate PEGDA, stir magnetically for 30min to form a homogeneous solution;
[0058] (2) Add the above solution into a beaker containing 50 g of isopropanol, and ultrasonically disperse for 2 hours;
[0059] (3) Add 20 g of NCM811 into the above-mentioned ultrasonically dispersed solution, stir overnight magnetically, and irradiate the slurry with a UV lamp during stirring until the isopropanol in the slurry is completely volatilized to obtain a coated and modified positive electrode material;
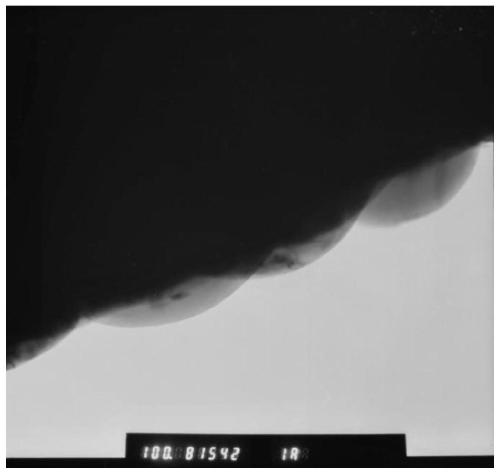
[0060] TEM observation was carried out on the above-mentioned coated and modified positive electrode material, and the results are shown in figure 1 , figure 1 TEM image of the coated modified cathode material prepared in Example 1. TEM characterization shows that a uniform coating layer can be observed on the surface of the material.
[0061] (4) Assemble lithium ion battery with the N...
Embodiment 2
[0066] (1) Weigh 0.16g LiClO 4 Add 0.008g initiator AIBN to 0.836g isobornyl acrylate IBOA, stir magnetically for 20min to form a homogeneous solution;
[0067] (2) Add the above solution into a beaker containing 50 g of ethanol, and disperse it ultrasonically for 2 hours;
[0068] (3) Add 18g of NCM811 into the above ultrasonically dispersed solution, stir magnetically at 80°C for 24h, until the ethanol in the slurry is completely volatilized, and then heat-treat the slurry at 100°C for 3h to prepare the modified NCM811 material;
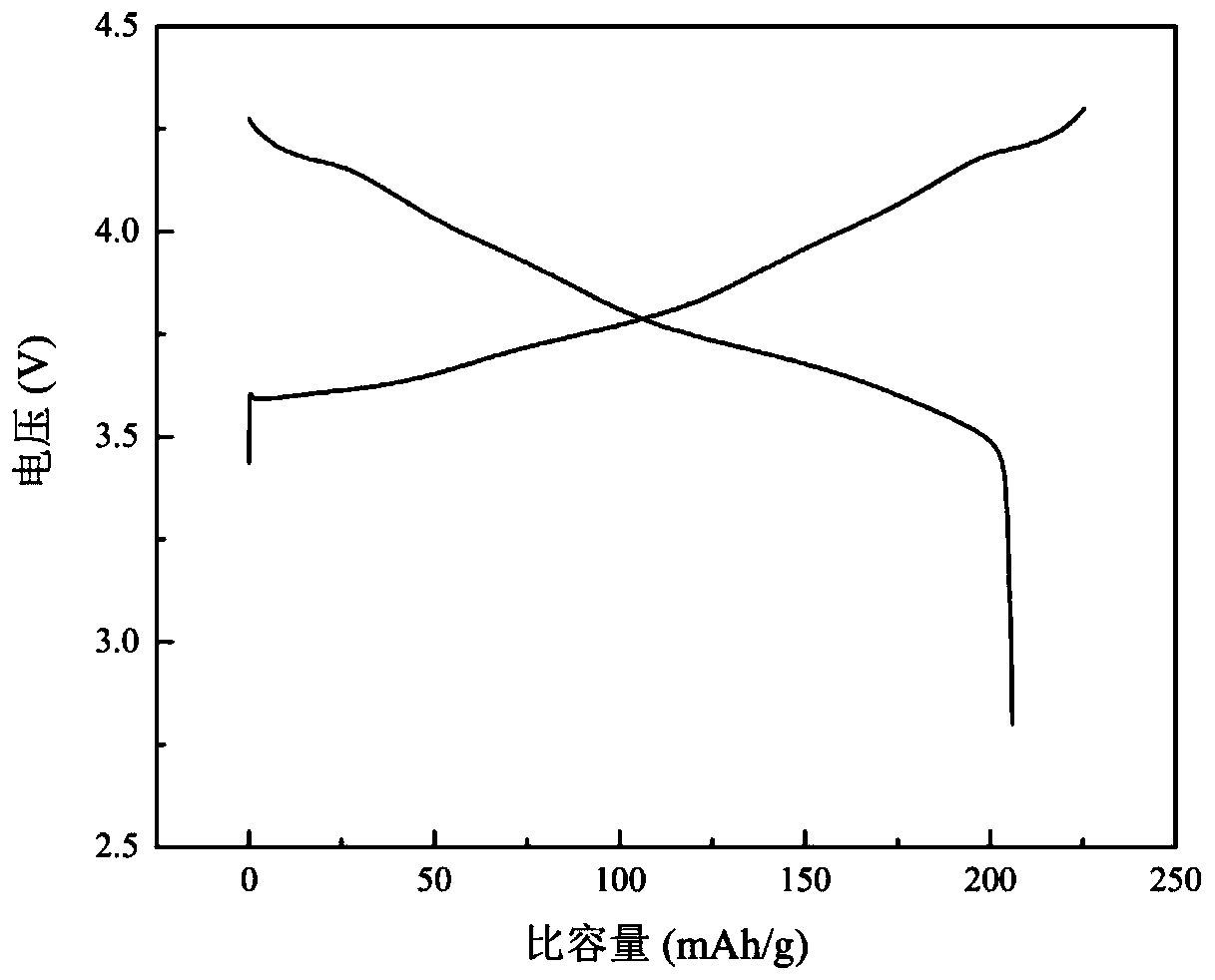
[0069] (4) Assemble a lithium-ion battery with the above-mentioned coated and modified NCM811 material, and test its electrochemical performance at room temperature (25°C) and a voltage range of 2.8 to 4.3V;
[0070] The test results show: the discharge capacity of the sample 0.1C of the comparative example is 204.0mAh / g, and the first Coulombic efficiency is 89.63%, while the discharge capacity of the sample 0.1C of Example 2 is 207.2mAh / g, and t...
Embodiment 3
[0072] (1) Weigh 0.12g LiFSI and 0.006g photoinitiator 184 and add to 0.877g pentaerythritol tetraacrylate PETEA, stir magnetically for 30min to form a homogeneous solution;
[0073] (2) Add the above solution into a beaker containing 50 g of ethanol, and magnetically stir and disperse for 3 h;
[0074] (3) Add 20g of NCM811 into the above-mentioned ultrasonically dispersed solution, stir overnight magnetically, and irradiate the slurry with a UV lamp during stirring until the ethanol in the slurry is completely volatilized;
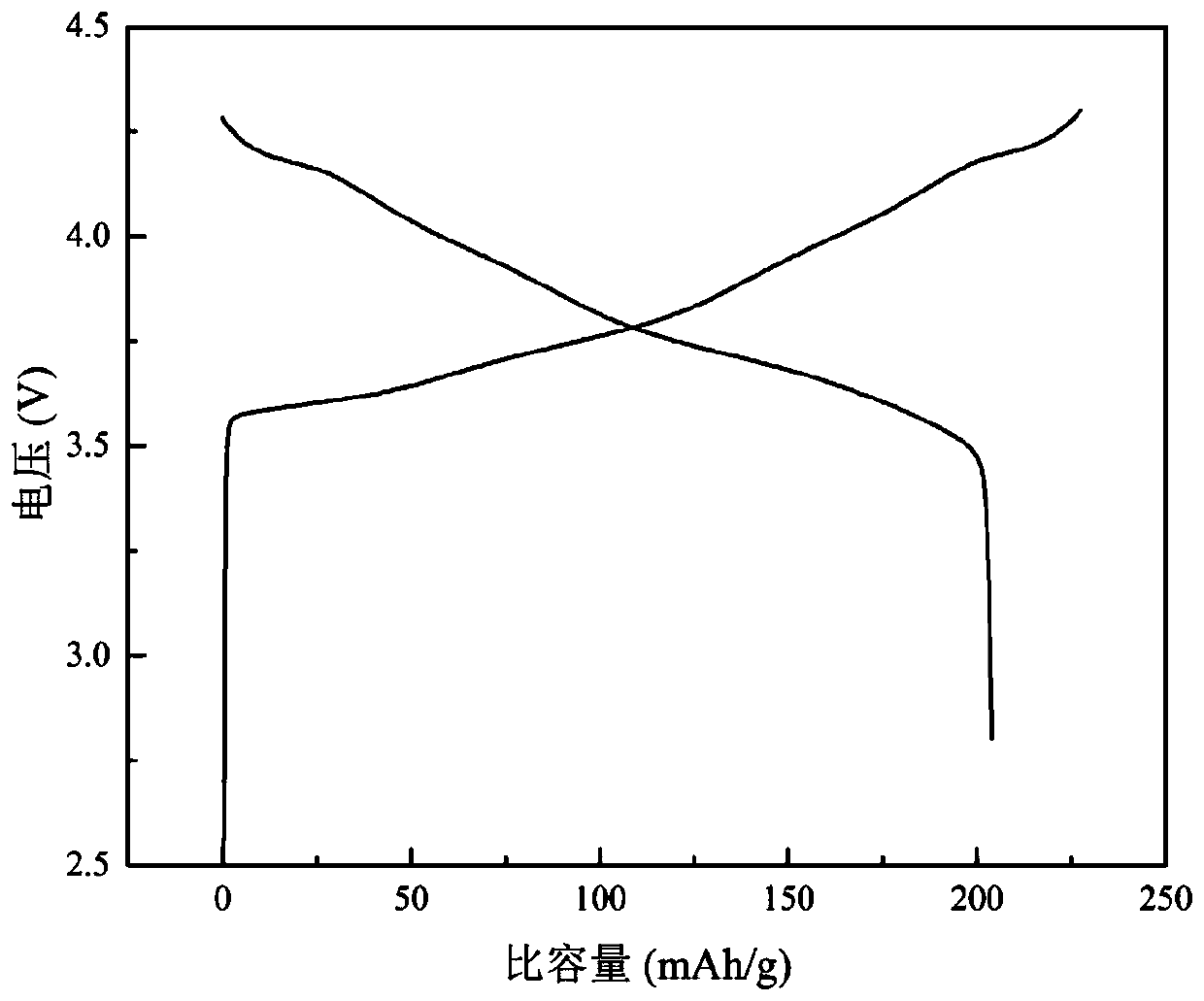
[0075] (4) Assemble a lithium-ion battery with the above-mentioned coated and modified NCM811 material, and test its electrochemical performance at room temperature (25°C) and a voltage range of 2.8 to 4.3V;
[0076] The test results show: the discharge capacity of the sample 0.1C of the comparative example is 204.0mAh / g, and the first coulombic efficiency is 89.63%, while the discharge capacity of the 0.1C sample of Example 3 is 205.4mAh / g, and the firs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Discharge capacity | aaaaa | aaaaa |
| Discharge capacity | aaaaa | aaaaa |
| Li-ion conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com