Method for preparing aldehyde by olefin hydroformylation
A technology of olefin hydroformyl and hydroformyl, which is applied in the direction of carbon monoxide reaction preparation, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor selectivity and low positive-to-isotropic ratio of products, and achieve lower reaction temperature and pressure, high Catalytic activity, the effect of improving catalyst activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Preparation of NiPd Bimetallic Phosphine-1NiPdP
[0029] Weigh 2.38g NiCl 2 ·6H 2 O, 2.31g Pd(NO 3 ) 2 , 1.56g NaH 2 PO 4 2H 2 O was placed in a beaker, and then 100 mL of deionized water was added, and stirred at a constant temperature at 70°C to dissolve and evaporate to obtain a dehydrated product. The product was dried at 80°C for 10 h and then ground to obtain a solid precursor, which was placed in a tube-type atmosphere resistance furnace, N 2 Calcined at 500°C for 1 h under protection, the crude product was taken out, washed with water and absolute ethanol three times, and vacuum-dried at 80°C for 12 h to obtain the final product NiPdP.
[0030] Preparation of nickel-palladium bimetallic phosphine-2NiPdP
[0031] Weigh 2.38g NiCl 2 ·6H 2 O, 2.31g Pd(NO 3 ) 2 , 2.34g NaH 2 PO 4 2H 2 O was placed in a beaker, and then 100 mL of deionized water was added, and stirred at a constant temperature at 70°C to dissolve and evaporate to obtain a dehydrated pr...
Embodiment 2
[0033] 1-Butene Hydroformylation
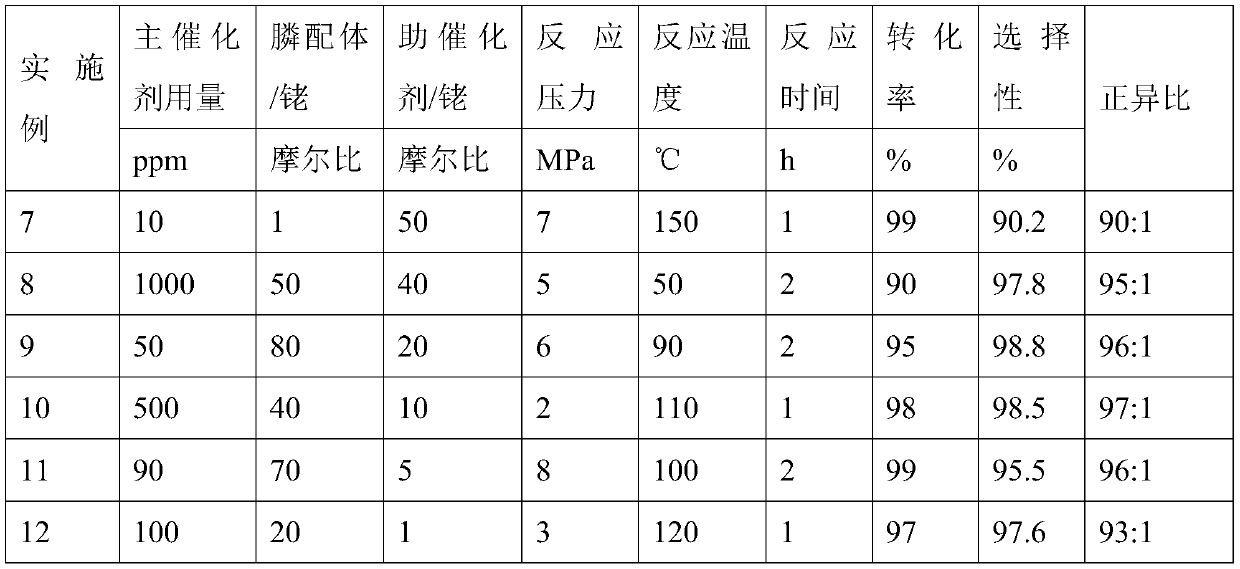
[0034] Rh(acac)(CO) 2 (0.35mmol, 90.5mg), phosphine ligand 3.5mmol, nickel-palladium bimetallic phosphine-20.35mmol, 1-butene 5.0L add in the 10.0L reactor, use synthesis gas (H 2 / CO=1) After replacing the reactor three times, stir with an electromagnetically driven mechanical stirrer, heat up to 110°C inside the reactor, feed synthesis gas until the total pressure is 2.0MPa, and react for 2 hours under this condition, the reaction process keep the pressure constant. After the reaction was over, the reaction kettle was taken out and placed in cold water for rapid cooling to stop the reaction. The reaction products were qualitatively analyzed by chromatography-mass spectrometer, quantitatively analyzed by gas chromatography, and quantified by calibration and normalization method. The conversion rate of olefins is 100%, the selectivity of aldehyde is 99.2%, and the positive-to-isotropic ratio is 96:1.
Embodiment 3~6
[0036] Hydroformylation reaction
[0037]According to the experimental method of Example 2, the cocatalyst uses nickel-palladium bimetallic phosphine-1, and 1-butene is replaced by propylene, 1-pentene, 1-hexene, and 1-heptene respectively for hydroformylation. The reaction results are shown in Table 1.
[0038] Table 1 hydroformylation reaction results
[0039] Example Olefin Conversion rate / % selectivity / % Isotropic ratio 3 Acrylic 99 95 90:1 4 1-pentene 96 97 95:1 5 1-Hexene 98 96 93:1 6 1-heptene 97 98 98:1
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com