Cyclic lipopeptide derivative and preparation and application thereof
A kind of derivative and cyclolipopeptide technology, which is applied to cyclolipopeptide derivatives and their preparation, and the application field of the cyclolipopeptide derivatives in antibacterial, which can solve the problems of low activity of plant pathogens and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
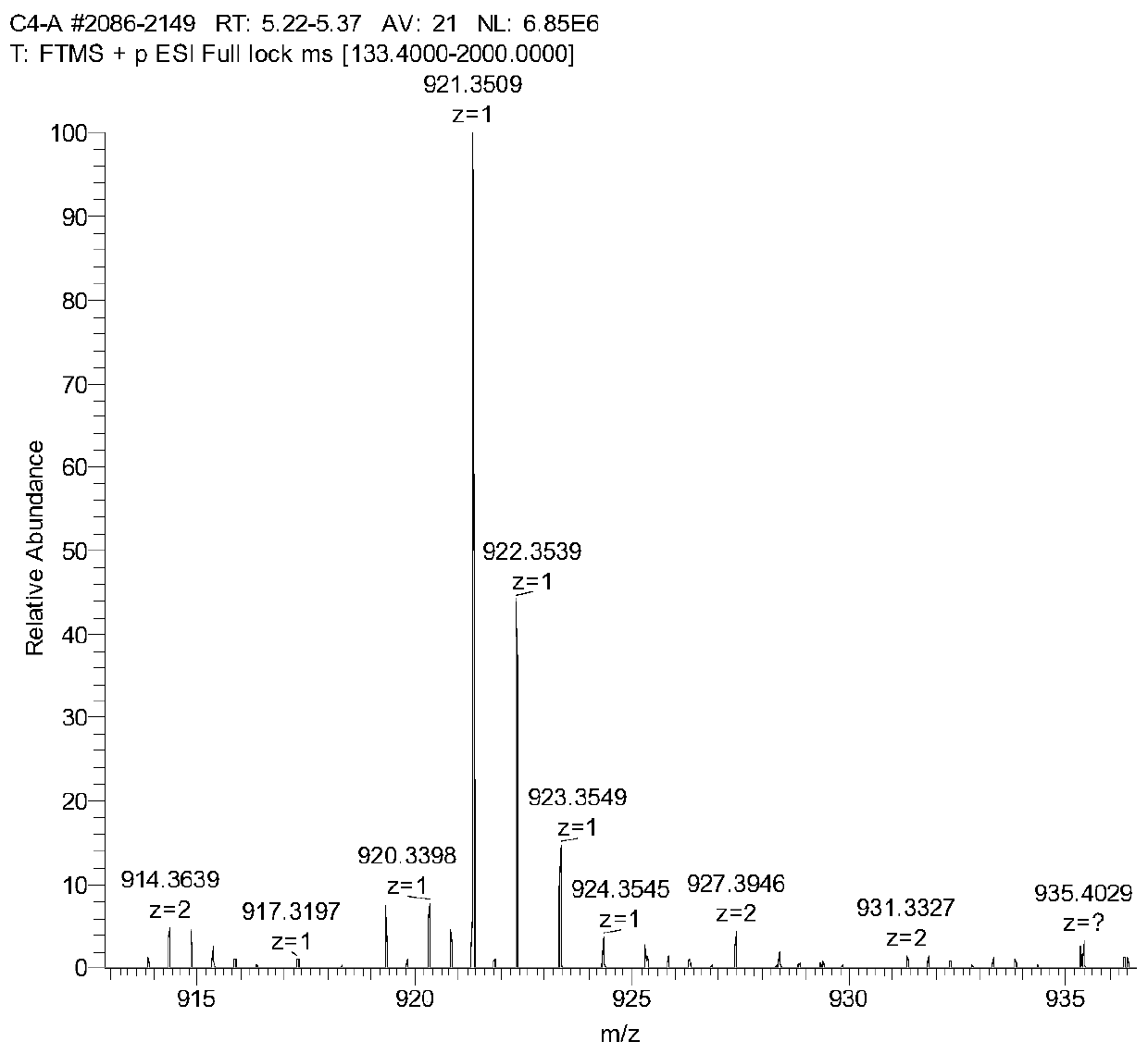
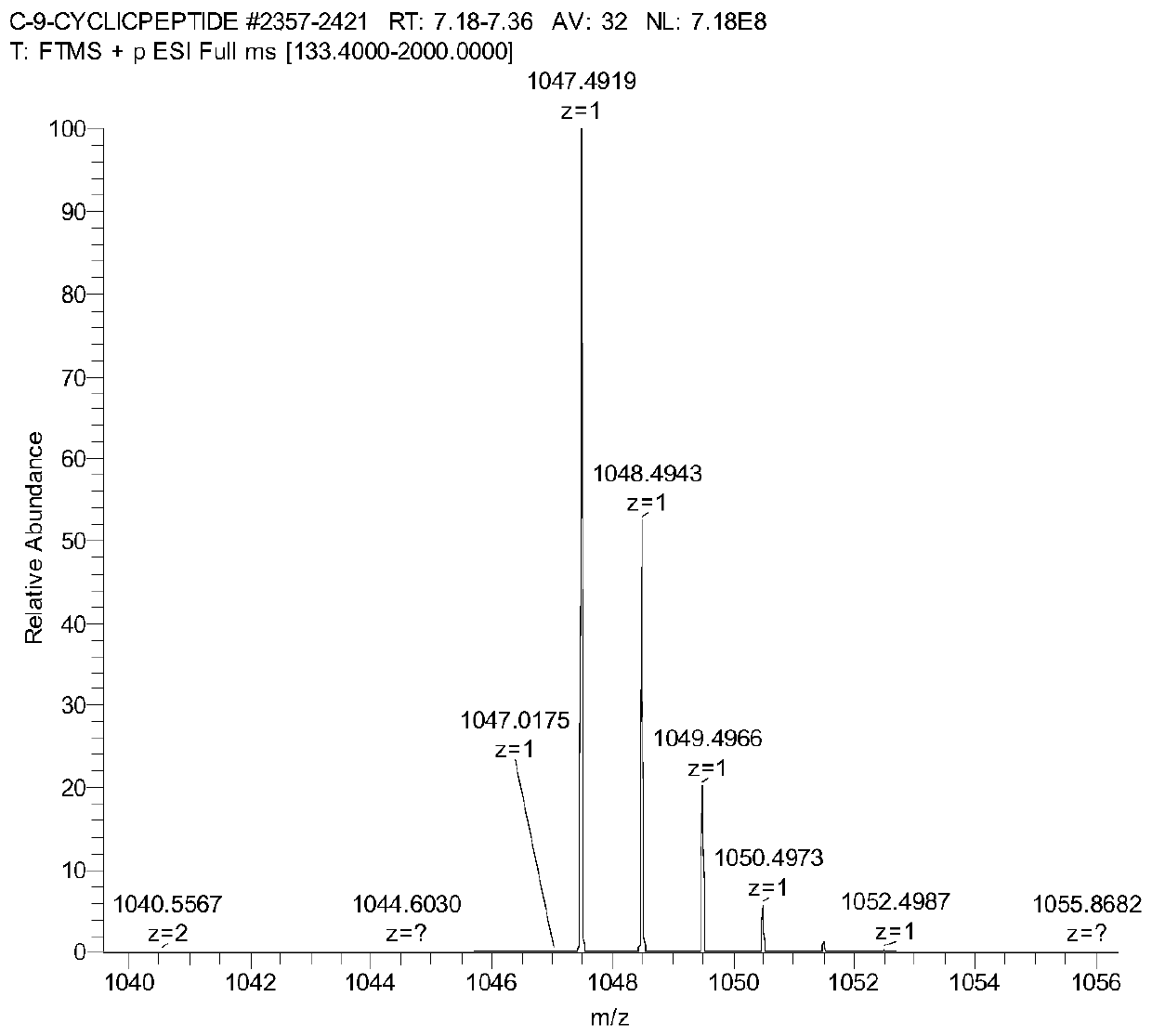
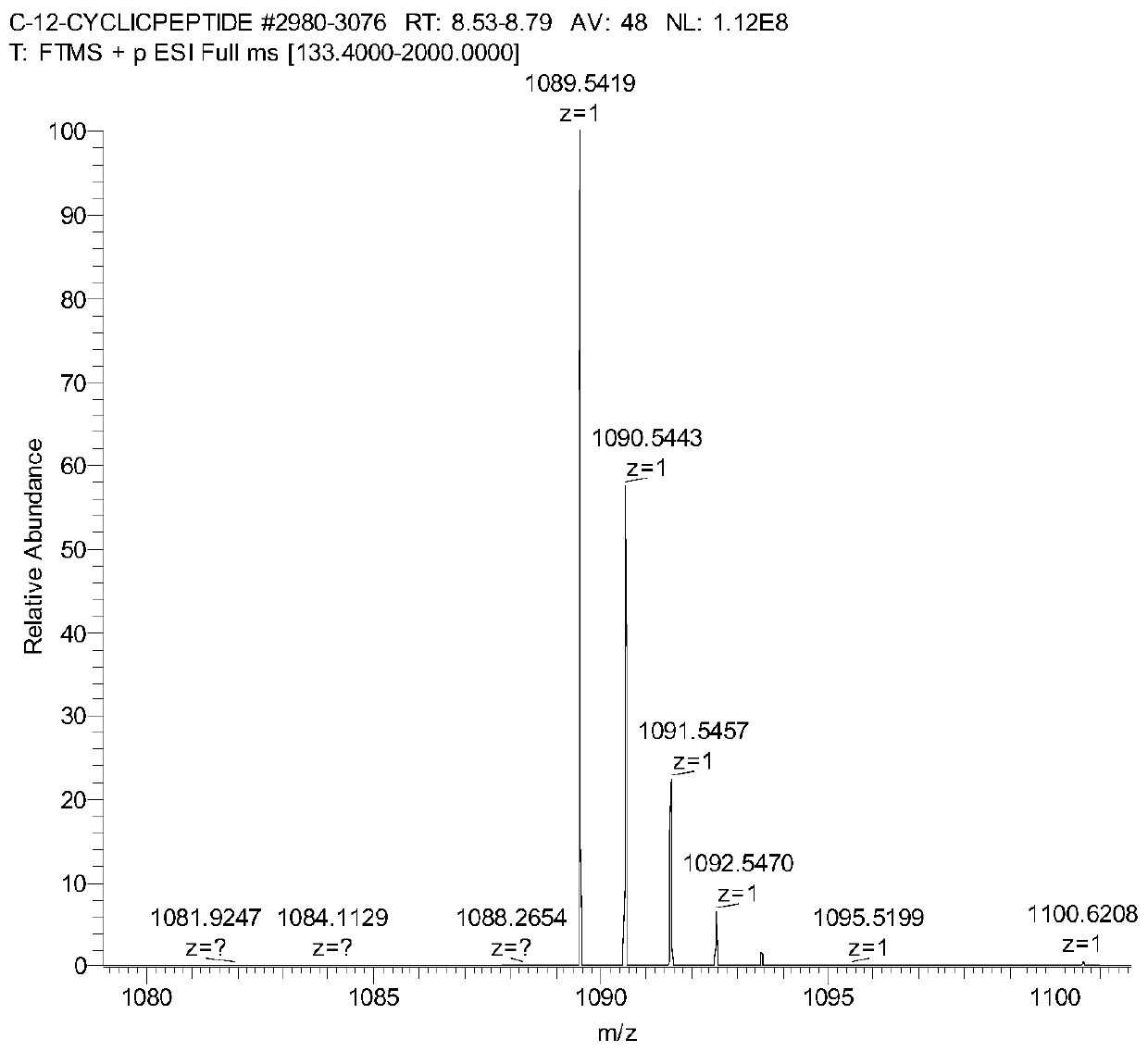
Image
Examples
preparation example Construction
[0127]The preparation method of the cyclic lipopeptide derivatives of the present invention can be prepared by the commonly used amino acid condensation method in the field. It can also be prepared according to the preparation method of the present invention. The preparation method of the invention comprises the synthesis of the cyclic peptide skeleton and the alkylation of the cyclic peptide skeleton. The synthesis of the cyclic peptide skeleton includes the preparation of the peptide chain (or amino acid chain) and the preparation of the cyclic peptide skeleton.
[0128] The preparation of the peptide chain (or amino acid chain) comprises the following steps: using 2-chlorotrityl chloride resin (2-Cl-Trt resin) as a carrier, passing the Fmoc-L-Asn-OH reagent in the first step Load Fmoc-L-Asn on the resin, and then remove Fmoc by deprotection treatment to obtain resin NH loaded with L-Asn 2 -L-Asn-(2-Cl-Trt resin); then replace Fmoc-L-Asn-OH with different amino acid reagen...
Embodiment 1
[0143] Synthetic method of cyclolipopeptide derivatives
[0144] 1: Synthesis of Cyclic Peptide Skeleton
[0145] 1.1 Synthesis of amino acid chains:
[0146] The synthesis starts with loading of L-Asn onto 2-chlorotrityl chloride (2-Cl-Trt) resin.
[0147] (1) 2-Chlorotrityl chloride resin (2-Cl-Trt resin) (1.1mmol / g, 0.1mmol) was transferred to a reaction vessel, washed with anhydrous DCM (3×5mL), and Swell in water DCM (5 mL) for 30 minutes. Fmoc-L-Asn-OH (0.3mmol) and DMF (Fmoc-L-Asn-OH:DMF 1:1) and DIPEA (0.6mmol) dissolved in DCM were added, and the mixture was incubated under N 2 Stir under atmosphere for 3 hours. After the coupling (Fmoc-L-Asn-(2-Cl-Trt resin)) was completed, the resin (Fmoc-L-Asn-(2-Cl- Trt resin)), dried under vacuum, and weighed to check the completion of this resin loading.
[0148] (2) Swell the resin obtained in the above steps in DMF (3×5mL), and treat it with DMF (10mL) containing 20% piperidine for 30 minutes. 5mL) to further wash the...
Embodiment 2
[0185] Activity Test of Cyclolipopeptide Derivatives
[0186]1. Activity detection method
[0187] 1.1 Bacteria experiment
[0188] All biological tests were performed with a modified broth microdilution test recommended by the Clinical and Laboratory Standards Institute (CLSI) that had been developed to determine in vitro antimicrobial activity with little modification.
[0189] All compounds were tested against different bacterial strains: Bacillus cereus, Bacillus subtilis, Staphylococcus epidermidis, Pseudomonas putida, Klebsiella pneumoniae ( Klebsiella pneumonia), Escherichia coli (E. coli).
[0190] Bacterial strains were grown overnight at 37°C in LB medium and bacterial growth was measured by turbidity as optical density at λ = 600 nm (OD600). Dilute the bacterial strain to 10 per ml 6 working solution of colony forming units. Compounds were dissolved in DMSO to form stock solutions. 96-well round-bottom polypropylene plates and 100 μl of diluted bacterial strai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com