Application of FGF-2 derived polypeptide to preparation of medicines for promoting cartilage repairing and/or treating degenerative arthritis
A FGF-2, 1. The technology of FGF-2, applied in the field of biomedicine, can solve the problem of cartilage regeneration activity that has not been reported in the literature, and achieve the effects of low immunogenicity, easy synthesis, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1, the solid-phase synthesis of peptide PDG6 and KED7
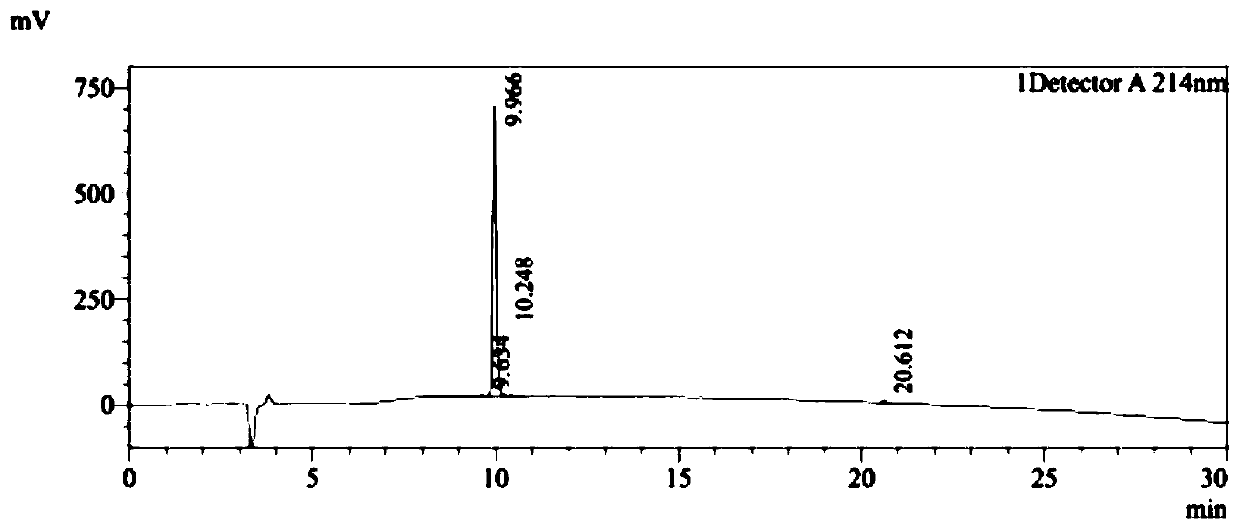
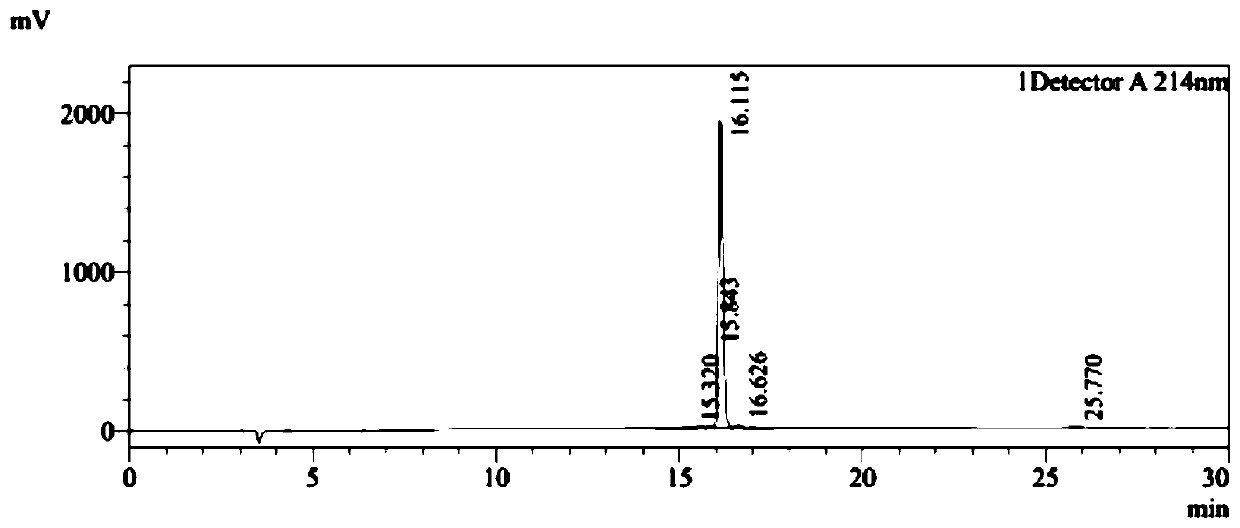
[0038] PDG6 (SEQ ID NO: 1) and KED7 peptide (SEQ ID NO: 2) were synthesized by a conventional solid-phase process, and the purity of the synthesized peptides was >95%. The HPLC detection profiles of the two peptides were as follows: Figure 1A and Figure 1B As shown, synthesize 500mg.
Embodiment 2
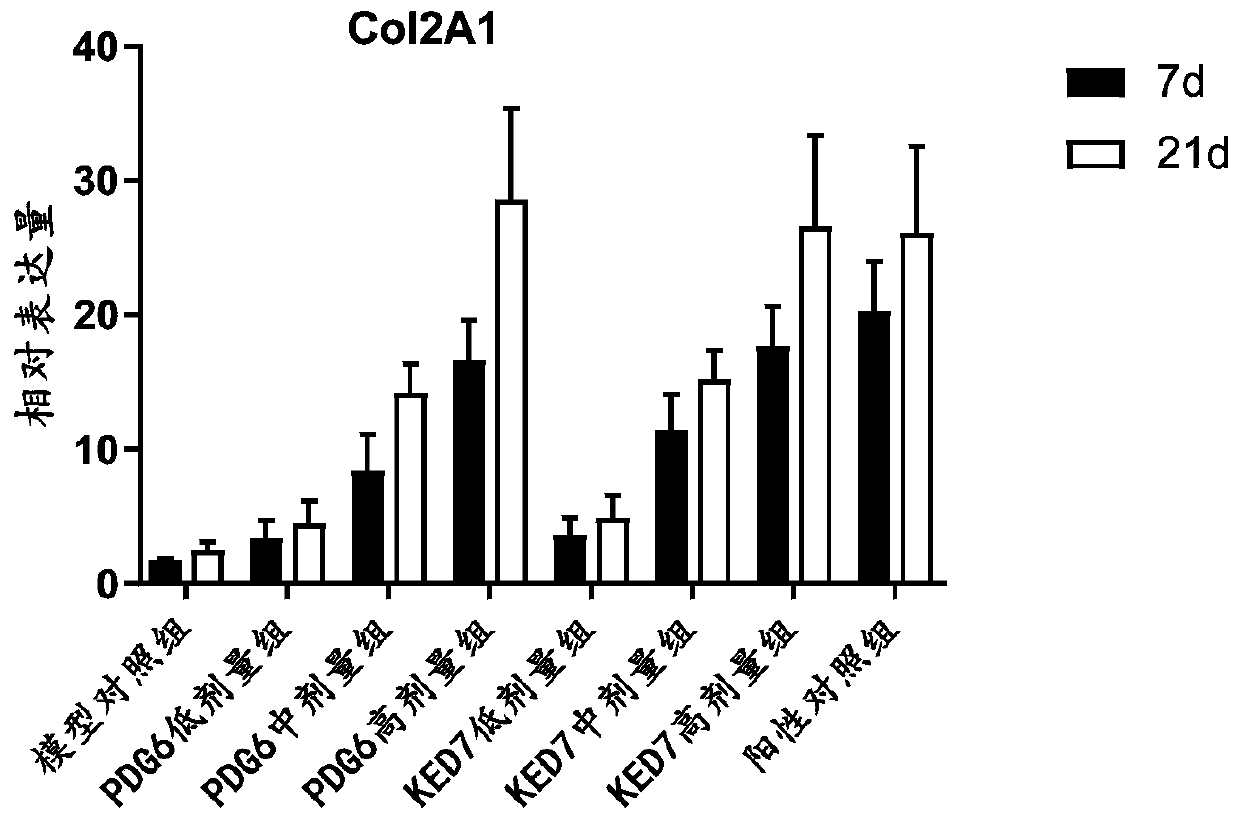
[0039] Example 2. The effect of peptide PDG6 and KED7 on the differentiation of mesenchymal stem cells in vitro
[0040] 1.1 Culture and passage of human mesenchymal stem cells: resuscitate human bone marrow mesenchymal stem cells (BMSCs, purchased from Guangzhou Saiye Biotechnology Co., Ltd.), add complete medium, and place at 37°C, 5% CO 2 cultured in a humidified incubator. On the second day after recovery, the recovered cells were replaced with fresh complete medium, and the cells were replaced with fresh complete medium every two days until the cells reached 80%-90% confluence, and then subcultured.
[0041] 1.2 Chondrogenic induction: The peptides PDG6 and KED7 were dissolved in phosphate buffered solution (PBS). Cells were resuspended in complete chondrogenic medium containing different concentrations of PDG6 or KED7 peptide (0.1 μM, 1 μM, and 10 μM) so that the concentration of BMSCs was 5.0 × 10 5 cells, and PBS was used as a negative control, and 1 μM human insulin...
Embodiment 3
[0047] Example 3. Effects of peptides PDG6 and KED7 on the proliferation of chondrocytes
[0048] 1.1 Chondrocyte culture: Cut the articular cartilage of 2-month-old New Zealand white rabbits under sterile conditions, cut into 1mm size, digest with 2mg / ml hyaluronidase for 45 minutes, 2mg / ml trypsin for 45 minutes, and 4mg / ml type II collagenase digested for 3 hours, washed and centrifuged (1500r / min) for 5 minutes, and the precipitate was placed in DMEM medium containing 15% fetal bovine serum for cultivation.
[0049] 1.2 Identification of chondrocytes: Take a small amount of primary chondrocyte smears and immunodetect with type I collagen antibody SABC, stained brownish yellow, indicating the secretion of type I collagen, and proved to be chondrocytes.
[0050] 1.3 MTS method to measure chondrocyte proliferation: chondrocytes were measured according to 2×10 4 Each well was inoculated in a 96-well plate, and different concentrations of peptides PDG6 and KED7 (0.1, 1, and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com