A kind of preparation method of icotinib key intermediate
A technology of icotinib and intermediates, which is applied in the field of drug synthesis, can solve the problems of a large number of acidic waste liquids, operation hazards, environmental hazards, etc., and achieves the effects of high reaction yield, avoiding operation hazards and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
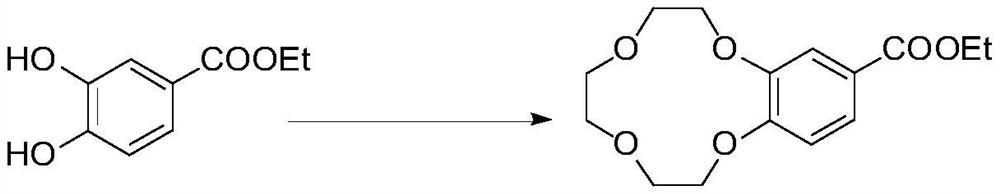
[0020] In the supercritical reactor, place triethylenedi(p-toluenesulfonate) 50g and 20g of ethyl 3,4-dihydroxybenzoate in the reactor, then start stirring after the whole system is vacuumized, to Ammonia gas is passed into the reactor to make the pressure in the reactor reach normal pressure, and then carbon dioxide is fed into the reactor, and the pressure in the reactor increases to 12MPa, and the carbon dioxide gradually becomes liquid, and triethylenedi(p-toluenesulfonate ) and ethyl 3,4-dihydroxybenzoate were fully dissolved, the temperature in the reactor was raised to 50°C, the reaction was stopped after a certain period of time, carbon dioxide was released slowly, and a turbid solid appeared in the reactor, and then 70 mL of methanol was added to it Mixed solution with 30mL of acetone, stirred at 0°C for 10min, filtered with suction and dried to obtain 27.2g of ethyl 3,4-(benzo-12-crown-4)benzoate, which can effectively avoid by-products of ester hydrolysis...
Embodiment 2
[0022]
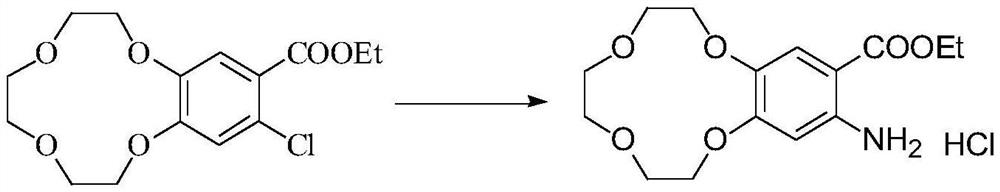
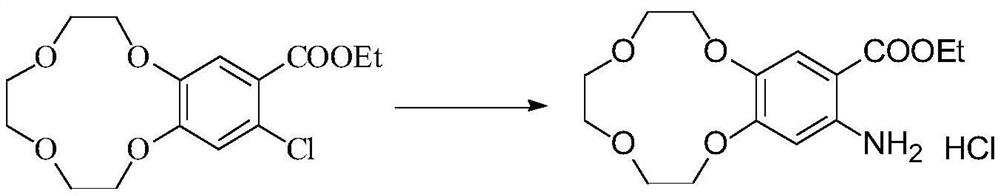
[0023] Dissolve 30g of ethyl 3,4-(benzo-12-crown-4)benzoate in 300mL of dichloroethane, stir at 40°C for 20min, then add N-chlorosuccinimide in three batches 15g, each batch of 5g at an interval of 1h, after adding the last batch of NCS, slowly raise the temperature to 55°C, after a period of reaction, TLC monitors the complete reaction of the raw materials, cool down to room temperature, filter the reaction solution, pour the filtrate into 1000mL of ice water, add Saturated sodium hydroxide solution 200mL, separate the organic phase, adjust the pH of the organic phase to be neutral with dilute hydrochloric acid solution, separate the organic phase again, dry with anhydrous magnesium sulfate and concentrate to obtain 6-chloro-3,4-( Benzo-12-crown-4) ethyl benzoate 30g, 1 H NMR (400Hz, DMSO-d 6 ):7.34(s,1H),6.95(s,1H),4.26(dd,J 1 =8.0Hz,J 2 =4.0Hz,2H),4.01(t,J 1 =4.0Hz,J 2 =4.0Hz, 2H), 3.98-3.96(m, 2H), 3.81(t, J 1 =4.0Hz,J 2 =4.0Hz, 2H), 3.69-3.66(m, 2H), 3....
Embodiment 3
[0025]
[0026] Dissolve 30g of ethyl 3,4-(benzo-12-crown-4)benzoate in 300mL of dichloroethane, stir at 40°C for 20min, then add 15g of N-chlorosuccinimide at one time , slowly raised the temperature to 60°C, after a period of reaction, TLC monitored the complete reaction of the raw materials, cooled to room temperature, filtered the reaction solution, poured the filtrate into 1000mL of ice water, added 200mL of saturated sodium hydroxide solution, separated the organic phase, the organic phase The pH was then adjusted to be neutral with dilute hydrochloric acid solution, and the organic phase was separated again, dried over anhydrous magnesium sulfate, and then concentrated to obtain 22 g of ethyl 6-chloro-3,4-(benzo-12-crown-4)benzoate, 1 H NMR (400Hz, DMSO-d 6 ):7.34(s,1H),6.95(s,1H),4.26(dd,J 1 =8.0Hz,J 2 =4.0Hz,2H),4.01(t,J 1 =4.0Hz,J 2 =4.0Hz, 2H), 3.98-3.96(m, 2H), 3.81(t, J 1 =4.0Hz,J 2 =4.0Hz, 2H), 3.69-3.66(m, 2H), 3.61(s, 4H), 1.28(t, J 1 =4.0Hz,J 2 =4.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com