Method for catalyzing ring-opening polymerization of cyclic ester by aza-aromatic ring
An aromatic ring-catalyzed cyclic ester and ring-opening polymerization technology, which is applied in the field of nitrogen-heteroaromatic catalyzed ring-opening polymerization of cyclic esters, and can solve problems such as poor catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
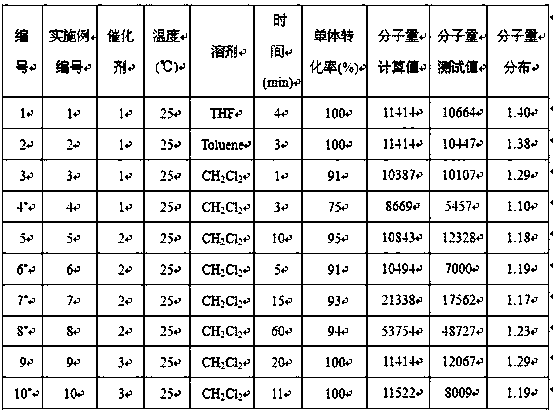
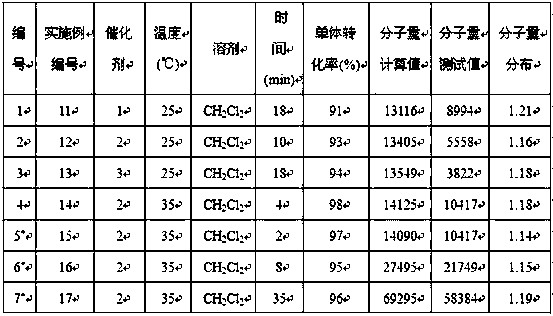
[0015] Example 1-10: Catalyst 1-3 Catalyzed performance research of ε-caprolactone ring-opening polymerization reaction
[0016] Among them, in Example 1-2, Catalyst 1 was used to carry out ring-opening polymerization of ε-caprolactone at the same temperature, different solvents, and the ratio of monomer, catalyst, and initiator being 100:1:1.
Embodiment 1
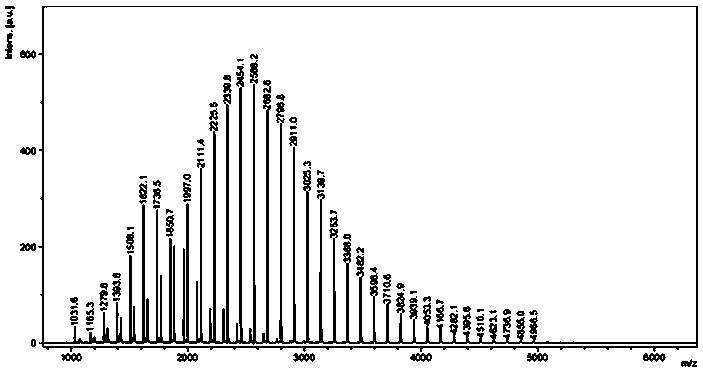
[0018] Under the condition of anhydrous and oxygen-free nitrogen atmosphere, absorb 5.8 mg (50 μmol) of the catalyst 1 liquid and dissolve it in 5 mL tetrahydrofuran solvent, and then add 0.02 mL n-butyllithium (2.5mol / L, 50 μmol) to it As an initiator, stir the reaction for 30 min; control the temperature at 25 °C and add 0.55 mL (5 mmol) ε-caprolactone monomer at a ratio of monomer:catalyst:initiator molar ratio of 100:1:1, control Stirring was continued for 4 min at 25 °C. During the reaction, by absorbing the reaction liquid, using 1 H NMR analysis monitors monomer conversion and makes calculations and records. Then 3.0 mL of benzoic acid (quencher) was added to terminate the reaction, and then 80 mL of methanol was added to completely precipitate the white polymer. The white polymer was filtered and washed, and finally the polycaprolactone product was obtained by drying. The results of the polymerization reaction are shown in Table 1 No. 1.
Embodiment 2
[0020] The solvent was toluene, and the stirring reaction was continued for 3 minutes, and the rest of the operations were the same as in Example 1. The results of the polymerization reaction are shown in Table 1 No. 2.
[0021] It can be seen that when the polymerization reaction is carried out in tetrahydrofuran and toluene solvent, the monomer conversion rate of 100% can be achieved in a short period of time, but the reaction rate carried out in tetrahydrofuran solvent is faster, and the relative molecular weight of the generated polymer product is The smaller the distribution value, the better the performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com