Genetic engineering stain for improving yield of isoprene and construction method and application thereof
A technology of genetically engineered bacteria and isoprene, applied in the field of genetic engineering, can solve the problems of insignificant improvement in catalytic efficiency, low catalytic efficiency, and bacterial toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1. The construction method of the genetically engineered bacterium that improves isoprene output.
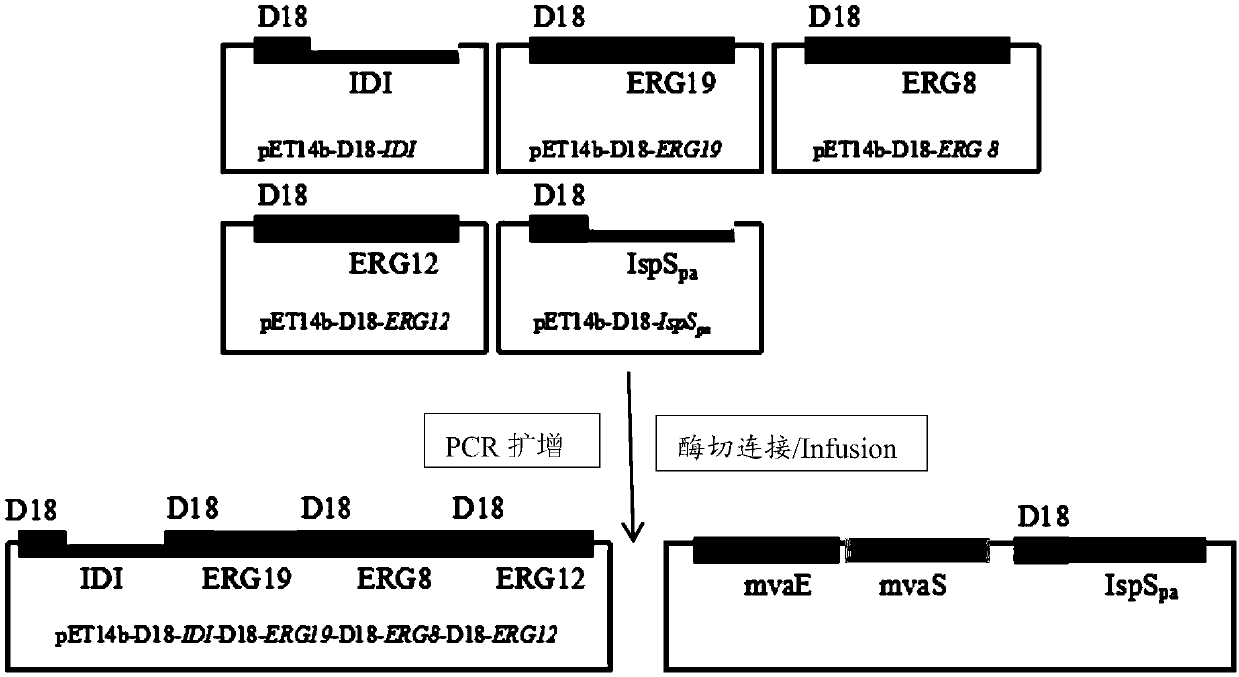
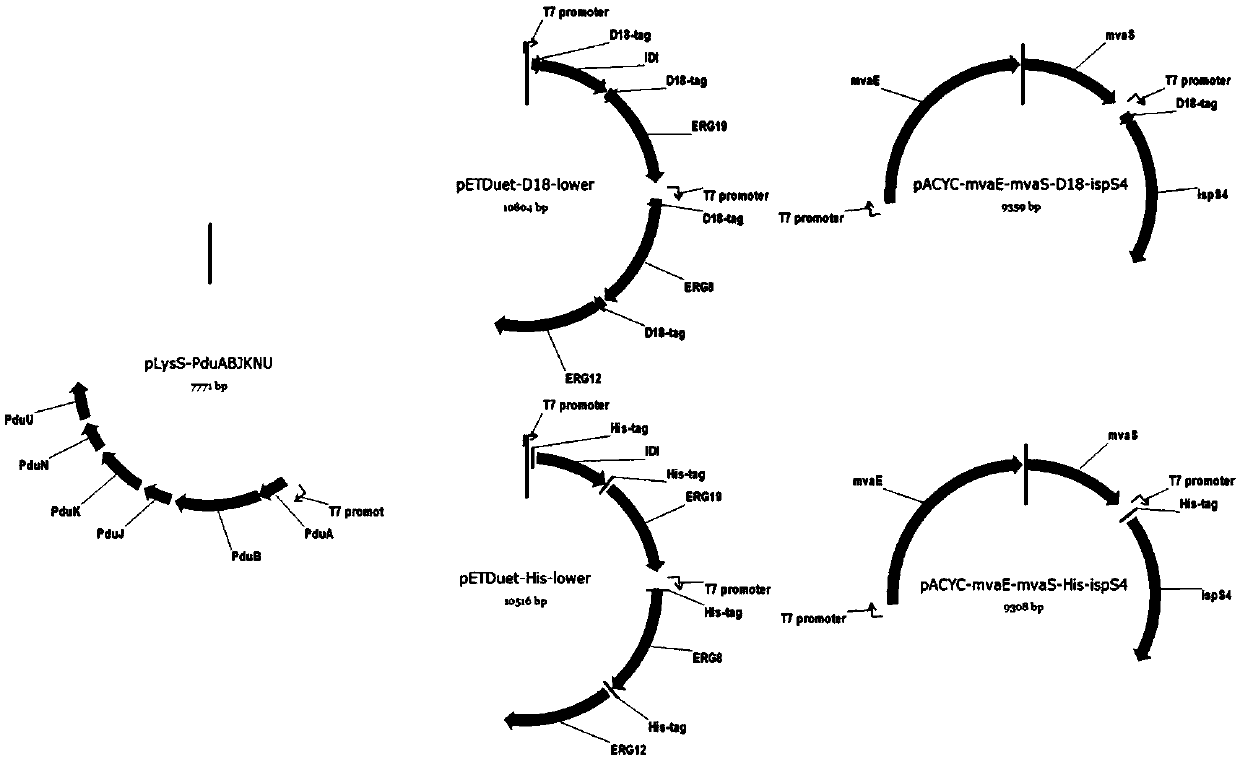
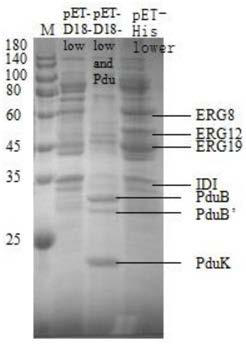
[0051] The genetically engineered bacterium prepared in this example to increase the production of isoprene overexpresses the mevalonate kinase gene ERG12 containing the D18-wrapped signal peptide sequence, the phosphomevalonate kinase gene ERG8 containing the D18-wrapped signal peptide sequence, and the Mevalonate pyrophosphate decarboxylase gene ERG19 with D18 wrapped signal peptide sequence, isopentenyl pyrophosphate isomerase gene IDI1 with D18 wrapped signal peptide sequence, isoprene synthase gene with D18 wrapped signal peptide sequence IspS pa , and recombinant plasmids containing PduA, PduB, PduJ, PduK, PduN and PduU gene clusters, and the starting strain is Escherichia coli. The amino acid sequence of the D18 wrapped signal peptide is shown in SEQ ID NO:1. The mevalonate kinase gene ERG12 is the mevalonate kinase gene ERG12 derived from Saccharomyce...
Embodiment 2
[0121] Example 2. Application of the genetically engineered bacteria prepared in Example 1 in fermentative production of isoprene.
[0122] This example illustrates the use of Example 1 to prepare the genetically engineered bacteria to produce isoprene through an in vitro enzyme-catalyzed reaction. The genetically engineered bacteria are induced to express proteosomes, and then the proteosomes are added to the in vitro enzymes. In the catalytic reaction system, the isoprene is prepared by incubating at room temperature. The specific method is as follows:
[0123] 1. Validation of Proteosomes
[0124] (1) The recombinant Escherichia coli constructed in Example 1 was inoculated in 100 mL LB medium, and the shaker was cultivated to OD at 37° C. at 180 rpm 600 0.6-1.0, add inducer IPTG to a final concentration of 0.4mM, and incubate at 30°C for 9 hours.
[0125] (2) Centrifuge the bacterial solution at 5000rpm for 10min, wash twice with Buffer A, add 10mL buffer A and 15mL BPER...
Embodiment 3
[0140] Example 3. Application of the genetically engineered bacteria prepared in Example 1 in fermentative production of isoprene.
[0141] This example illustrates the fermentation and synthesis of isoprene by using the prepared genetically engineered bacteria in a shake flask.
[0142] Inoculate the activated engineered Escherichia coli into the LB liquid culture solution containing the corresponding antibiotics at a ratio of 1:100, culture at 37°C with shaking at 180rpm, when the OD 600 When the temperature is 0.6-0.8, add IPTG with a final concentration of 0.4mM to the bacterial solution, and then transfer to 30°C and continue culturing at 180rpm. After the engineered strains were induced and cultured for 24 hours, 1 mL of the headspace gas was taken and quantitatively detected by gas-phase GC. The detection method was referred to Yang J, Xian M, Su S, Zhao G, Nie Q, JiangX, et al. -isoprene using hybrid MVA pathway and isoprene synthase in E. coli. PLoS one. 2012;7:e3350...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com