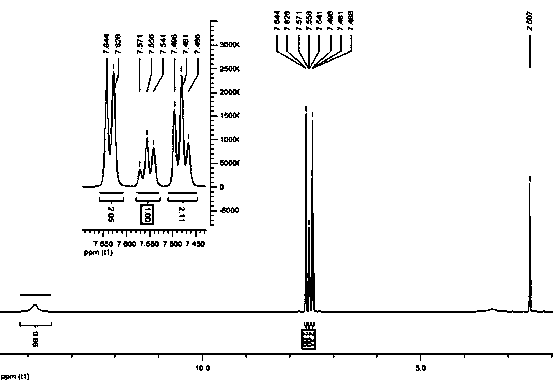
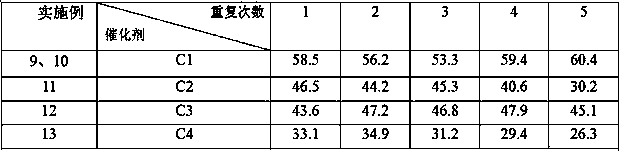
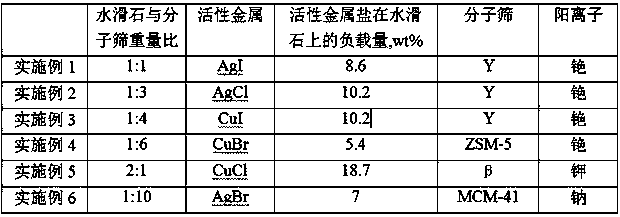
Catalyst for synthesizing phenylpropiolic acid and preparation method and application of catalyst
A technology of phenylpropiolic acid and catalyst, which is applied in the direction of catalyst activation/preparation, organic compound preparation, carboxylate preparation, etc. It can solve the problems of restricting reaction economy, loss of catalyst and additives, short service life of catalyst, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Prepare a 1mol / L silver nitrate solution, impregnate 50g of hydrotalcite in 20mL of silver nitrate solution, dry for 24 hours, and then bake at 200°C for 5 hours. Prepare 1mol / L sodium iodide solution, immerse the hydrotalcite loaded with silver nitrate in 20mL sodium iodide solution, dry for 24 hours, and then bake at 120°C for 24 hours.
[0048] (2) Prepare a 0.2 mol / L cesium carbonate solution, use the cesium carbonate solution to carry out ion exchange with silver iodide-loaded hydrotalcite, the solid-to-liquid ratio is 1:10 g / L, and exchange at 70°C for 2 hours. It was then filtered, washed and dried at 40°C. Repeat once. Finally, it was fired at 150° C. for 2 hours.
[0049] (3) Prepare a 1 mol / L cesium chloride solution, use the cesium chloride solution to perform ion exchange with NaY molecular sieves, the solid-liquid ratio is 1:5 g / L, and exchange at 70°C for 2 hours. Then filter, wash, dry at 40°C, and finally bake at 550°C for 2 hours. Repeat once. ...
Embodiment 2
[0052] (1) Prepare 2mol / L silver acetate solution, impregnate 50g hydrotalcite in 20mL silver acetate solution, dry for 24 hours, and then bake at 200°C for 5 hours. Prepare 2mol / L potassium chloride solution, immerse the hydrotalcite loaded with silver acetate in 20mL potassium chloride solution, dry for 24 hours, and then bake at 180°C for 8 hours.
[0053] (2) Prepare a 1 mol / L cesium carbonate solution, use the cesium carbonate solution to carry out ion exchange with silver chloride-loaded hydrotalcite, the solid-to-liquid ratio is 1:5 g / L, and exchange at 50°C for 6 hours. It was then filtered, washed and dried at 40°C. Repeat 2 times. Finally, it was fired at 150° C. for 2 hours.
[0054] (3) Prepare a 0.1mol / L cesium chloride solution, use the cesium chloride solution to perform ion exchange with NaY molecular sieves, the solid-liquid ratio is 1:10 g / L, and exchange at 40°C for 12 hours. Then filter, wash, dry at 40°C, and finally bake at 400°C for 12 hours. Repeat ...
Embodiment 3
[0057] (1) Prepare 1.5mol / L cuprous nitrate solution, immerse 50g hydrotalcite in 20mL cuprous nitrate solution, dry for 24 hours, and then bake at 200°C for 5 hours. A 1.5 mol / L cesium iodide solution was prepared, and the hydrotalcite loaded with cuprous nitrate was immersed in 20 mL of the cesium iodide solution, dried for 24 hours, and then calcined at 200°C for 2 hours.
[0058] (2) Prepare 0.6 mol / L sodium carbonate solution, use sodium carbonate solution to carry out ion exchange with cuprous iodide-loaded hydrotalcite, the solid-liquid ratio is 1:8 g / L, and exchange at 40°C for 10 hours. It was then filtered and dried at 40°C. Repeat once. Finally, it was fired at 150° C. for 2 hours.
[0059] (3) Prepare a 0.5mol / L cesium chloride solution, use the cesium chloride solution to perform ion exchange with NaY molecular sieves, the solid-liquid ratio is 1:6 g / L, and exchange at 70°C for 2 hours. It was then filtered, dried at 40°C, and finally calcined at 600°C for 4 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com