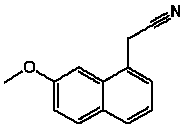
Preparation method of 7-methoxy-1-naphthylacetonitrile
A technology of methoxyl and naphthaleneacetonitrile, which is applied in the field of preparation of 7-methoxyl-1-naphthaleneacetonitrile, which can solve the problems of high production cost, high price, and inability to reuse
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 50.0g (0.25mol) of 7-methoxy-3,4-dihydro-1-naphthaleneacetonitrile and 350ml of toluene into a 500ml four-neck flask, stir to dissolve; add 42.5g (0.34mol) of allyl methacrylate , stirred for 10 minutes; then added 5% ruthenium / carbon 2.5g; heated to 80°C, stirred and reacted for 4hrs; sampling GC analysis: the normalized content of raw materials was 0.5%, and the reaction was completed;
[0030] After the reaction is finished, cool to room temperature, filter with suction, wash with a small amount of toluene, and reuse the catalyst; combine the filtrate and lotion, remove the solvent under reduced pressure until dry, add 300ml of 65% ethanol aqueous solution to the residue, heat to 65°C to dissolve, cool , cooled to 0°C, stirred and crystallized for 1hr; suction filtered, washed with 50% ethanol aqueous solution, and dried to obtain 47.6g of the product 7-methoxy-1-naphthaleneacetonitrile, with a content of 99.3% and a yield of 96.2%; the product was analyzed by NMR...
Embodiment 2~5
[0032] The following examples 2 to 5 are all operated according to the steps of example 1, only the catalyst ruthenium carbon is reused different times, and the implementation results are shown in the following table:
[0033] Embodiment 2~5 experimental result
[0034] Example serial number Ruthenium Carbon Reuse Times Response time (hr) Product content (%) Yield (%) 2 the second time 4.0 99.2 95.1 3 the third time 5.0 99.0 95.4 4 the fourth time 4.0 99.1 96.5 5 the fifth time 5.0 99.4 96.3
Embodiment 6
[0036] Add 50.0g (0.25mol) of 7-methoxy-3,4-dihydro-1-naphthaleneacetonitrile and 350ml of absolute ethanol into a 500ml four-necked flask, stir to dissolve; add 42.5g (0.34 mol), stirred for 10 minutes; then added 5% ruthenium / carbon 2.5g; heated to 70°C, stirred and reacted for 6hrs; sampling GC analysis: the normalized content of raw materials was 0.6%, and the reaction was over;
[0037] After the reaction is finished, cool to room temperature, filter with suction, wash with a small amount of ethanol, and reuse the catalyst; combine the filtrate and lotion, remove the solvent under reduced pressure until dry, add 300ml of 65% ethanol aqueous solution to the residue, heat to 65°C to dissolve, cool, Cool to 0°C, stir and crystallize for 1 hr; filter with suction, wash with 50% aqueous ethanol, and dry the solid to obtain 46.8 g of the product 7-methoxy-1-naphthaleneacetonitrile with a content of 99.5% and a yield of 94.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com