Cycloastragenol crystal form B and preparation method thereof
A technology of cycloastragalus and crystal form, which is applied in the field of organic chemical drug preparation, can solve the problems of unstable three-membered ring structure, complicated process, complicated purification steps of the final product and the like
Pending Publication Date: 2020-07-07
LUNAN PHARMA GROUP CORPORATION
View PDF6 Cites 1 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
[0004] At present, cycloastragenol is usually prepared by chemically hydrolyzing astragaloside IV. However, the three-membered ring structure on astragaloside IV is extremely unstable, and many by-products are easily formed.
Chinese patent CN104817610A uses sulfuric acid to hydrolyze astragaloside IV to prepare cycloastragenol, but the reaction requires a high-temperature and high-pressure reactor, and the purification steps of the final product are complicated; Chinese patent CN103880910A reports the preparation of cycloastragenol by a redox method, But its process is relatively complicated; in addition, patent CN105734109A and patent CN105566434A both use a variety of hydrolytic enzymes to hydrolyze astragaloside IV to prepare cycloastragenol
However, the existing literature has not yet reported the research on the related crystal forms of cycloastragenol
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
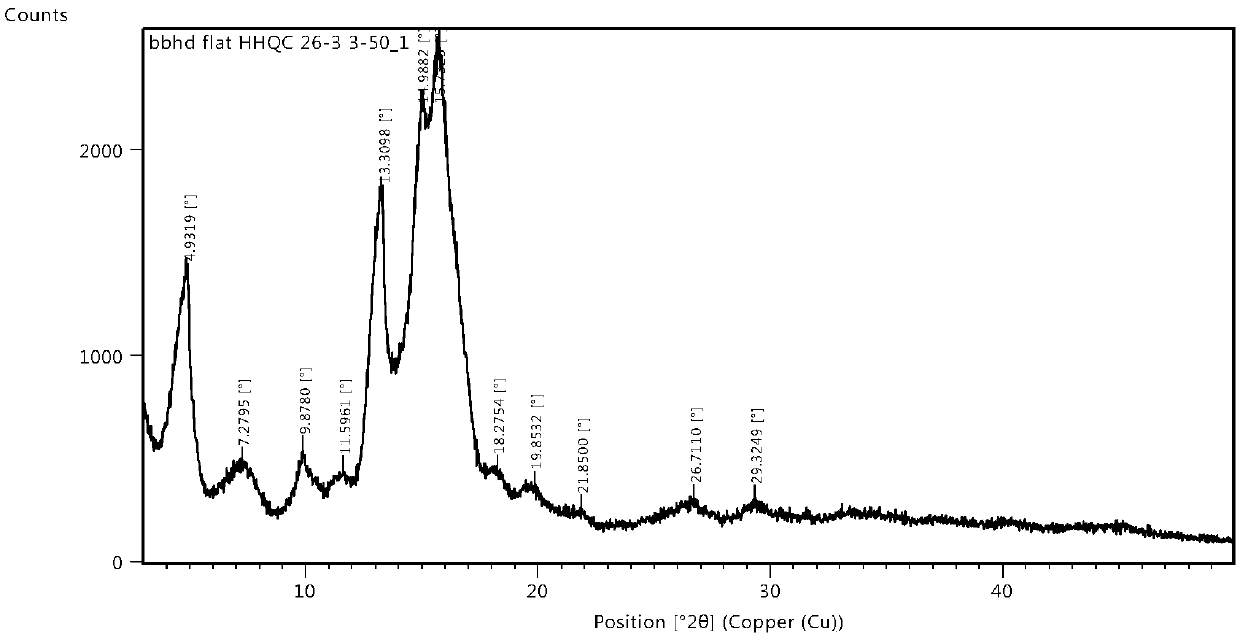
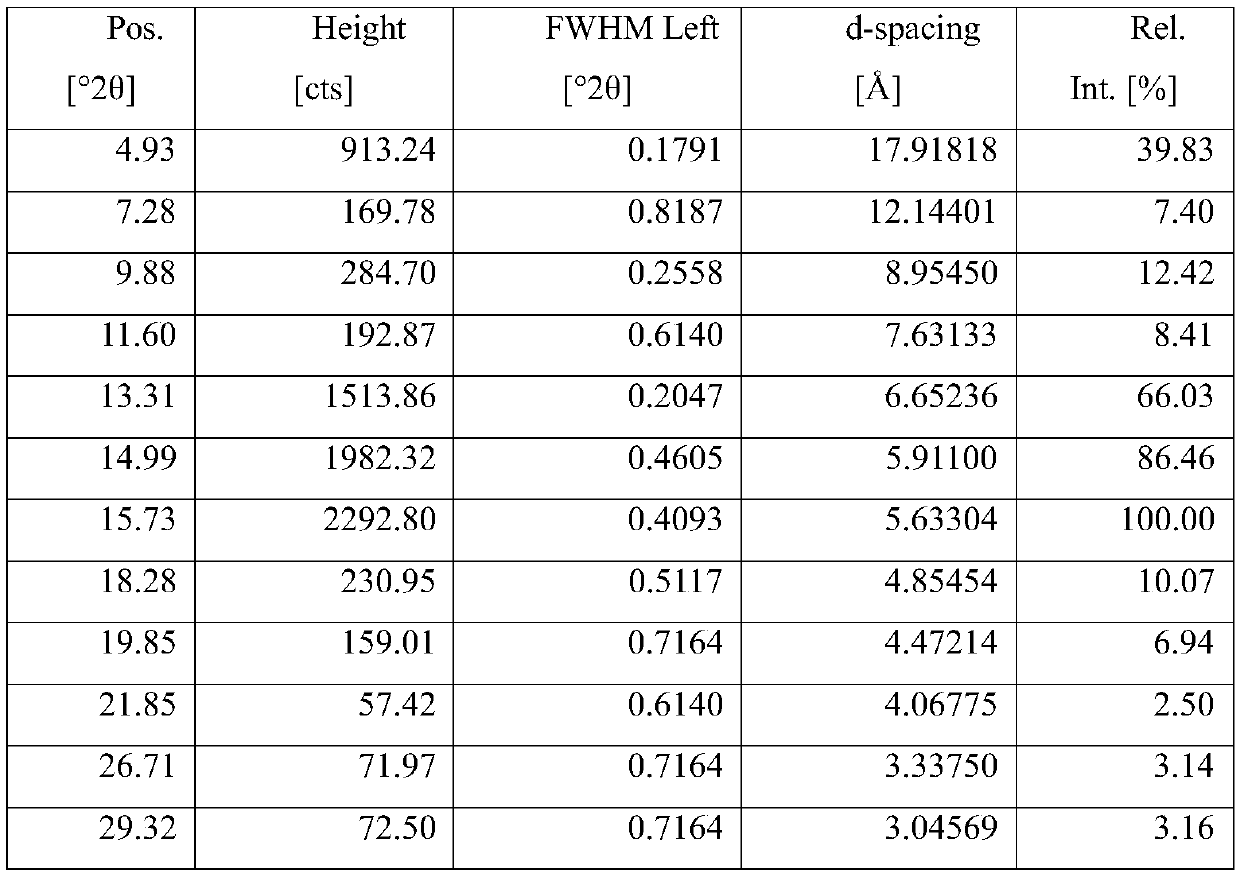
[0034] Add 250 mg cycloastragenol crystal form A into 6 ml of a mixed solution of methanol and methyl tert-butyl ether (volume ratio 1:5), heat to reflux, and after reflux for 2 to 4 hours, filter while hot to collect the filtrate, seal with a parafilm, tie Well, stand for crystallization for 2-4 days, filter, collect the solid, and dry under reduced pressure at 50°C to obtain a crystalline solid, yield: 96.3%, HPLC purity: 99.95%.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
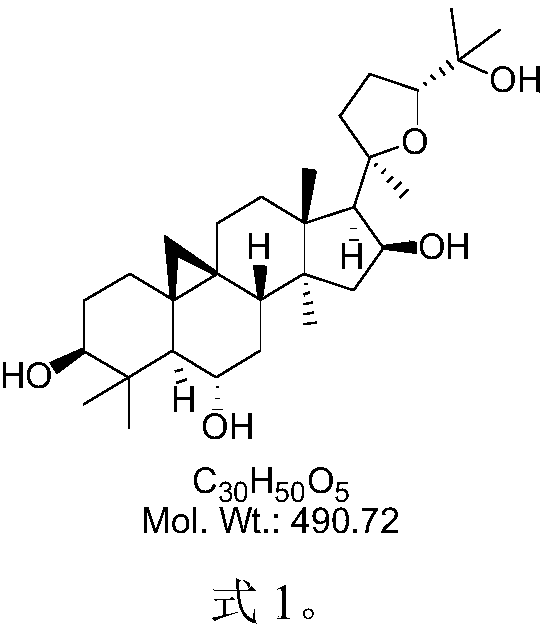
The invention provides a cycloastragenol crystal form B as well as a preparation method and application thereof. The cycloastragenol crystal form B provided by the invention has higher purity and better chemical stability, and a better dissolution and release effect can be achieved when the crystal form B is prepared into a pharmaceutical preparation, and meanwhile, the preparation method of cycloastragenol is simple, convenient and easy to industrialize.
Description
technical field [0001] The invention belongs to the technical field of organic chemical drug preparation, and in particular relates to cycloastragenol crystal form B, a preparation method and application thereof. Background technique [0002] Astragaloside IV (astragaloside IV) is a representative saponin component of the traditional Chinese medicine Astragalus membranaceus, and cycloastragenol (CAG) is a saponin of astragaloside IV. Cycloastragenol is the main hydrolysis metabolite of astragaloside in the intestinal tract and the component absorbed into the blood. It has a relatively small molecular weight and strong lipophilicity, which is conducive to biofilm penetration and gastrointestinal absorption to achieve better bioavailability. A large number of modern medical studies have proved that astragaloside IV can reduce the damage of ischemia-reperfusion brain tissue by enhancing the body's ability to scavenge oxygen free radicals, inhibiting peroxidation, and reducing ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07J53/00A61K31/58A61P39/06
CPCC07J53/004A61P39/06C07B2200/13
Inventor 张朝花翟立海郭立红胡长恺
Owner LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com