Synthetic method of aryl sulfone compound containing C (sp2)-S bond
A synthesis method and technology for sulfone compounds, applied in the direction of organic chemistry, etc., can solve the problems of selectivity problem, functional group compatibility, unfavorable industrial application, unfriendly environment, etc., and achieve a simple and efficient preparation method, which is beneficial to industrial application and easy to use in raw materials. the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]
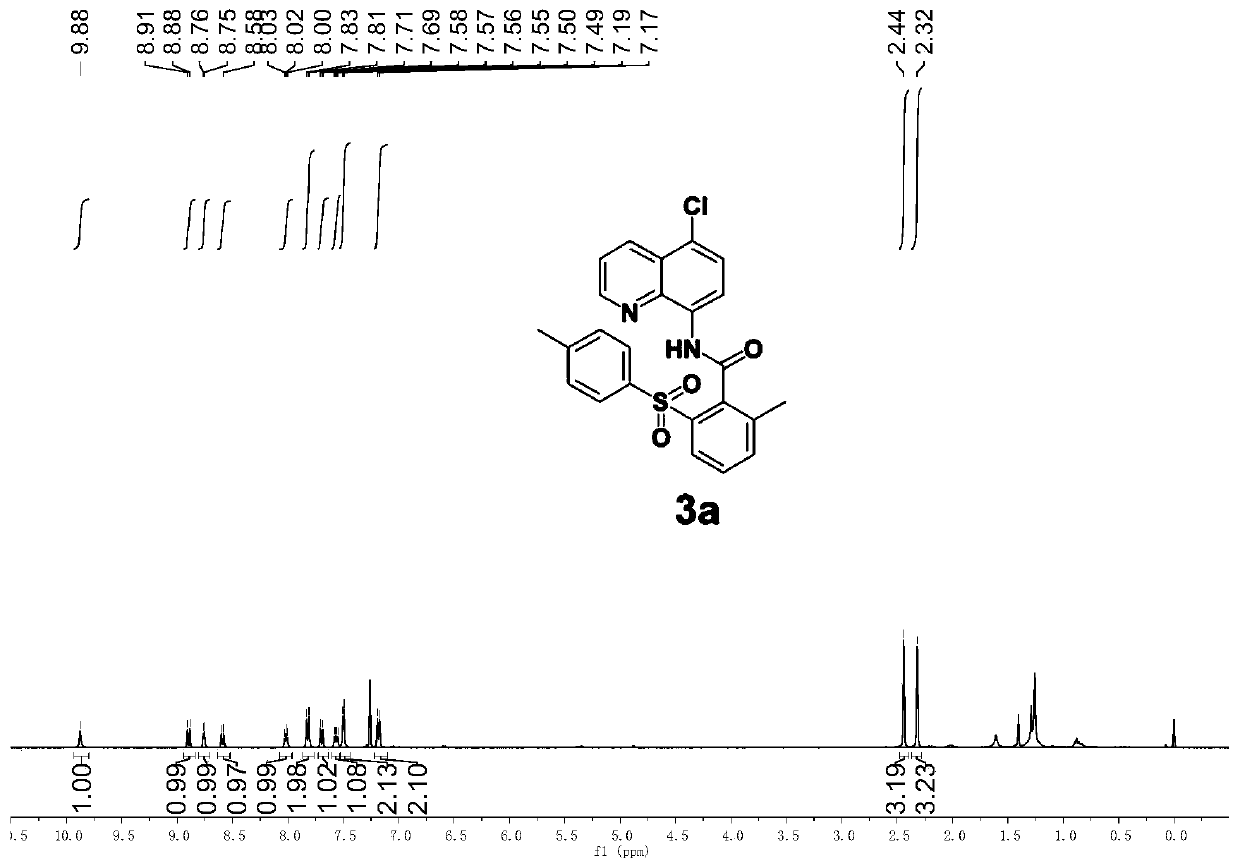
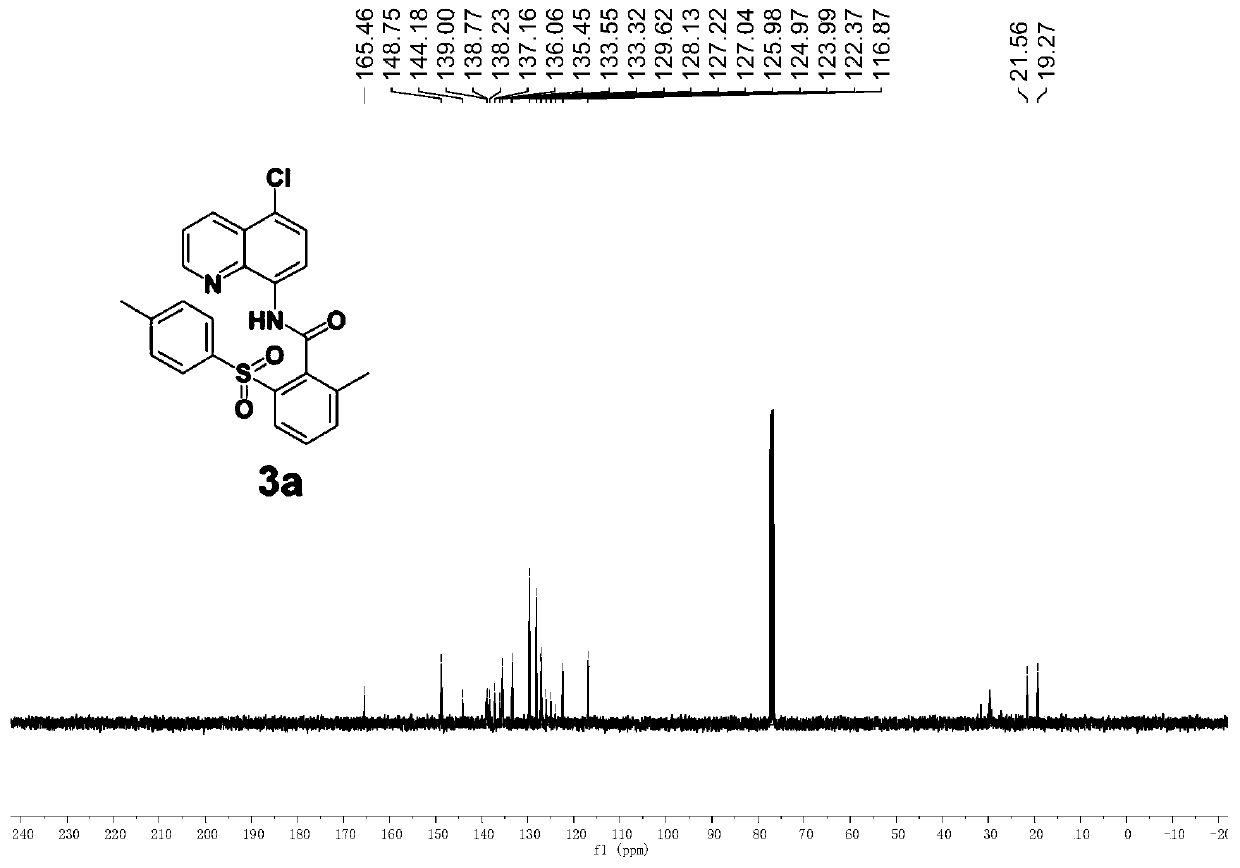
[0061] Add stir bar, DMF (1mL), 1a (59.4mg, 0.2mmol), 2a (106.9mg, 0.6mmol), copper (II) acetate (7.2mg, 0.02mmol), benzoic acid (7.4 mg, 0.03mmol), potassium pivalate (56mg, 0.4mmol), and the reaction system was placed in an oil bath at 80°C for 8h. After the reaction was completed, it was cooled to room temperature, diluted with ethyl acetate (5 mL), washed with water (3 × 20 mL), and then washed with brine (20 mL), the organic phases were combined and washed with anhydrous Na 2 SO 4 After drying and filtration, the filtrate was concentrated and separated by silica gel column chromatography (petroleum ether: ethyl acetate) to obtain a white solid 3a (72.2 mg, 80%, purity greater than 95%). The reaction yield is as high as 80%, and a product with a purity greater than 95% can be obtained by using a simple separation and purification method, indicating that the reaction method has the advantages of high reaction yield and good selectivity.
[0062] Product testin...
Embodiment 2
[0065]
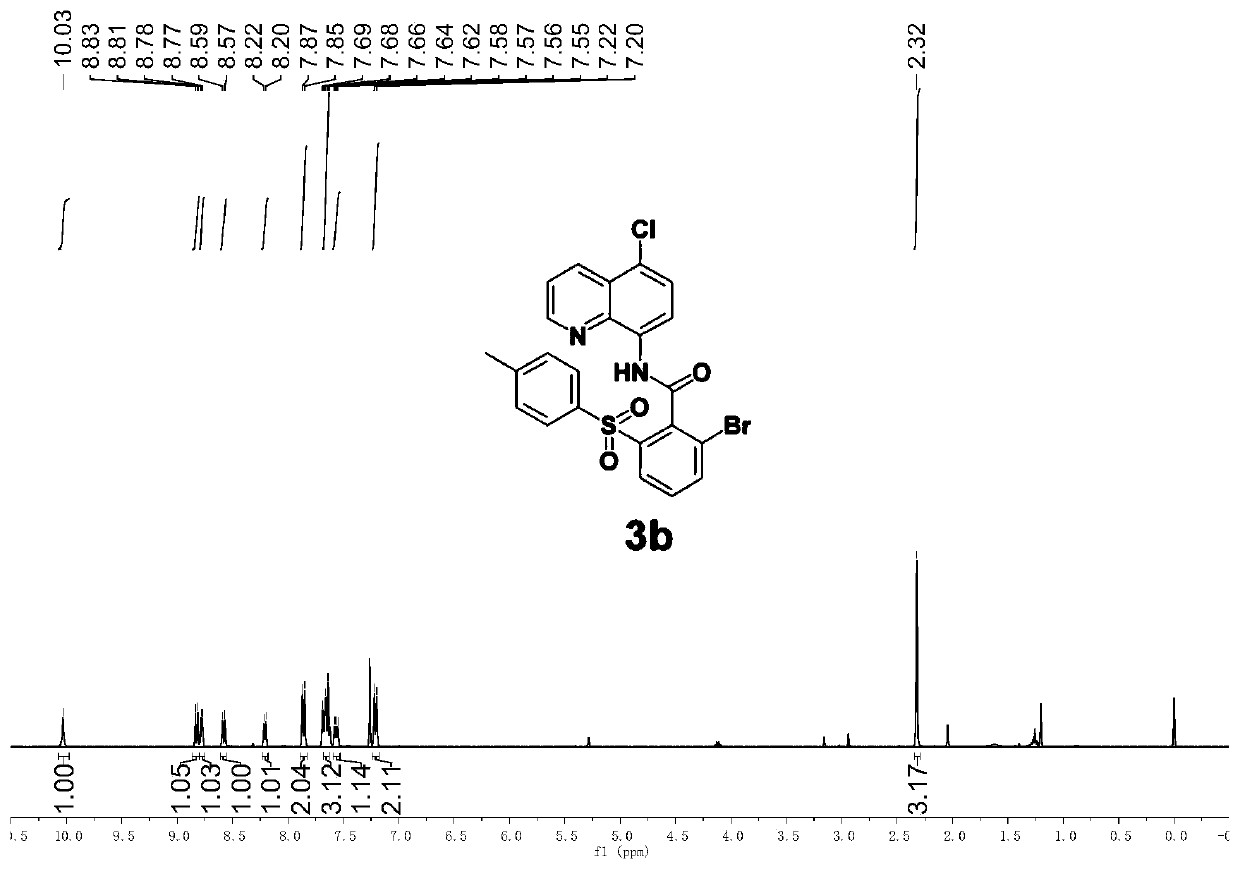
[0066] Add stir bar, DMF (1mL), 1a (72.3mg, 0.2mmol), 2a (106.9mg, 0.6mmol), copper (II) acetate (7.2mg, 0.02mmol), benzoic acid (7.4 mg, 0.03mmol), potassium pivalate (56mg, 0.4mmol), and the reaction system was placed in an oil bath at 80°C for 8h. After the reaction was completed, it was cooled to room temperature, diluted with ethyl acetate (5 mL), washed with water (3 × 20 mL), and then washed with brine (20 mL), the organic phases were combined and washed with anhydrous Na 2 SO 4 After drying and filtering, the filtrate was concentrated and separated by silica gel column chromatography (petroleum ether: ethyl acetate) to obtain a white solid 3b (64.5 mg, 63%, purity greater than 95%).
[0067] Product testing data are as follows: 1 H NMR (400MHz, CDCl 3 )δ10.03(s,1H),8.82(d,J=8.4Hz,1H),8.78(d,J=4.0Hz,1H),8.58(d,J=8.5Hz,1H),8.21(d, J=7.5Hz, 1H), 7.86(d, J=8.1Hz, 2H), 7.68–7.63(m, 3H), 7.56(dd, J=8.5, 4.2Hz, 1H), 7.21(d, J=8.2 Hz, 2H), 2.32(s, 3H). 13 C N...
Embodiment 3
[0069]
[0070] Add stir bar, DMF (1mL), 1a (71.8mg, 0.2mmol), 2a (106.9mg, 0.6mmol), copper (II) acetate (7.2mg, 0.02mmol), benzoic acid (7.4 mg, 0.03mmol), potassium pivalate (56mg, 0.4mmol), and the reaction system was placed in an oil bath at 80°C for 8h. After the reaction was completed, it was cooled to room temperature, diluted with ethyl acetate (5 mL), washed with water (3 × 20 mL), and then washed with brine (20 mL), the organic phases were combined and washed with anhydrous Na 2 SO 4 After drying and filtration, the filtrate was concentrated and separated by silica gel column chromatography (petroleum ether: ethyl acetate) to obtain a white solid 3c (52.41 mg, 51%, purity greater than 95%).
[0071] Product testing data are as follows: 1 H NMR (400MHz, CDCl 3 )δ9.59(s,1H),8.60(d,J=8.1Hz,2H),8.44(d,J=8.5Hz,1H),8.23(d,J=6.7Hz,1H),7.92(d, J=8.1Hz, 2H), 7.63(s, 2H), 7.54(d, J=8.3Hz, 1H), 7.41(d, J=8.1Hz, 3H), 7.25–7.19(m, 2H), 7.16( t, J=7.4Hz, 2H), 7.09(d, J=7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com