Fluorescent probe molecule for detecting diaphorase based on rhodamine derivative and preparation method and application thereof
A technology of fluorescent probe and diaphorase, which is applied in fluorescence/phosphorescence, chemical instruments and methods, and material analysis through optical means, can solve the problem of rare diaphorase and achieve good stability of physical and chemical properties , good application prospects and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
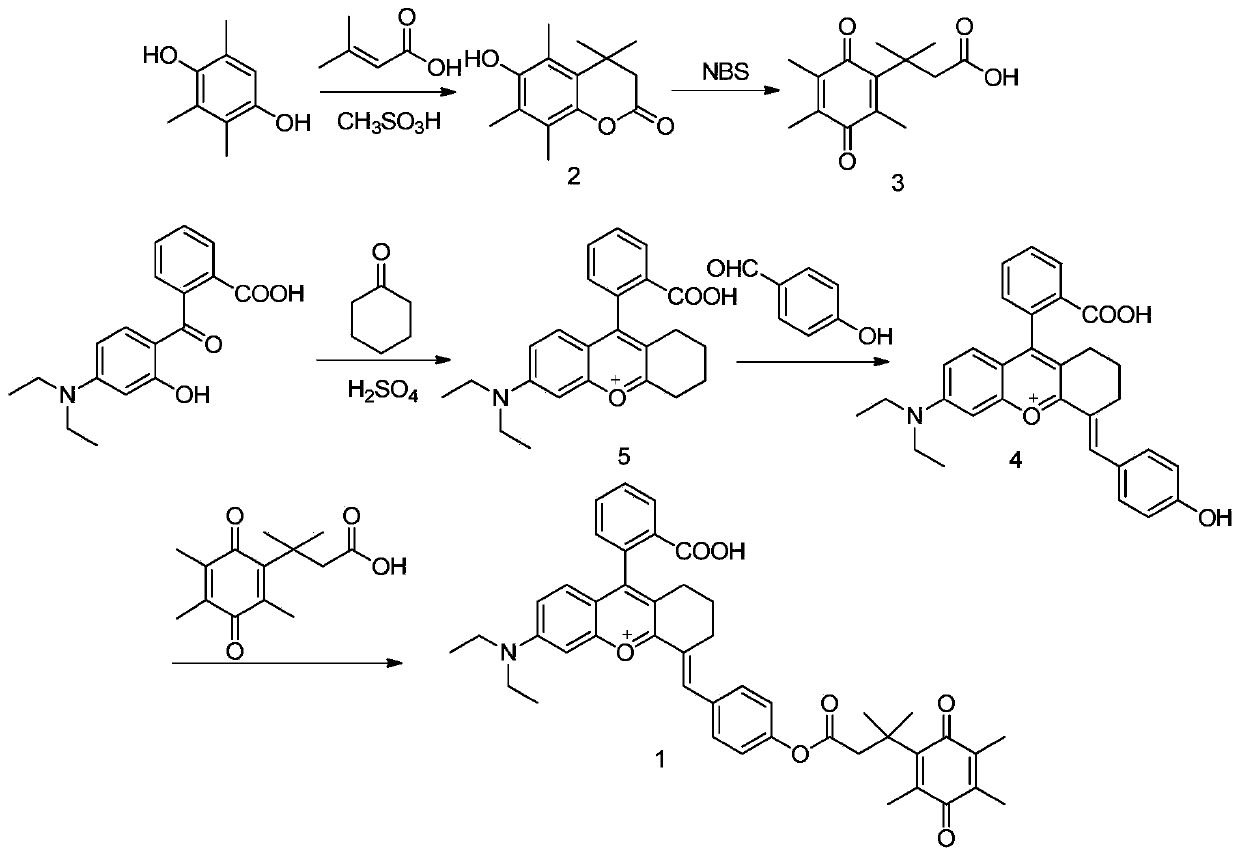
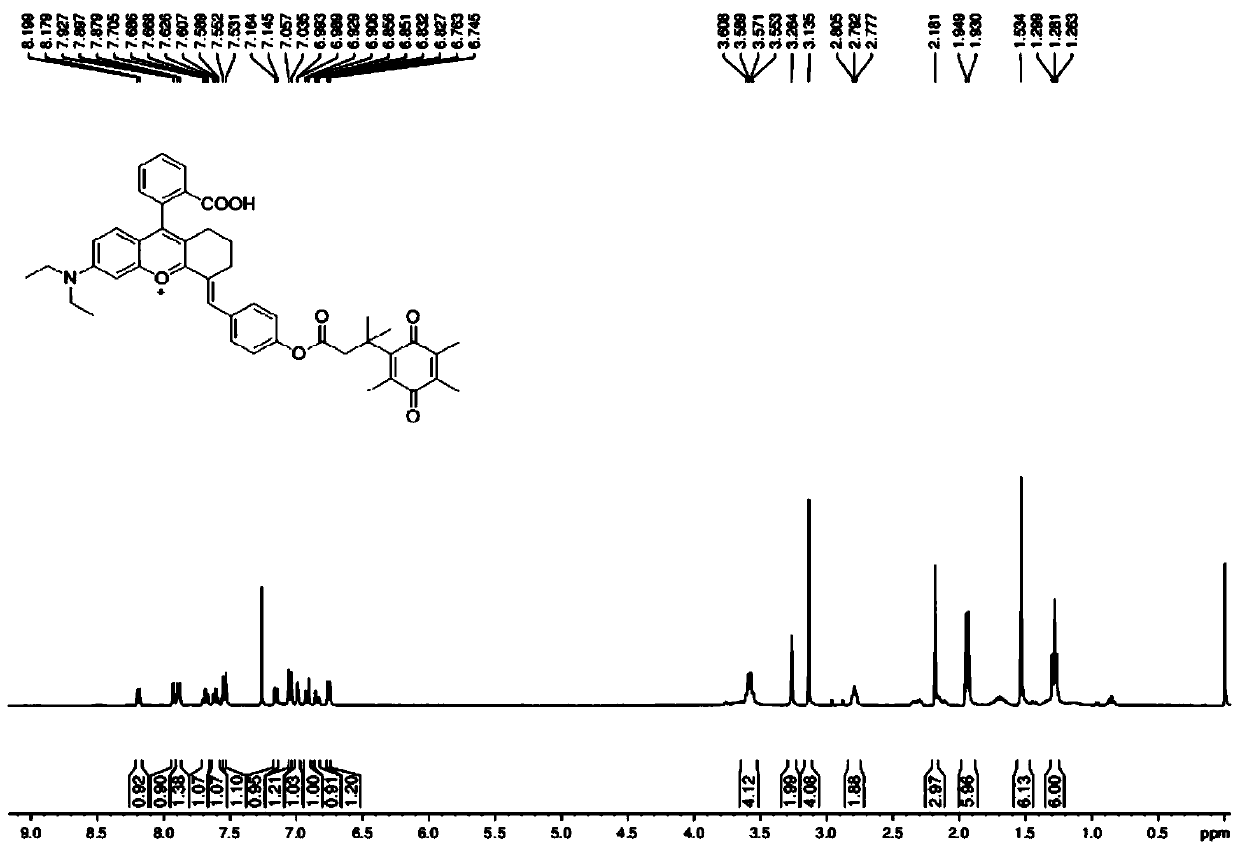
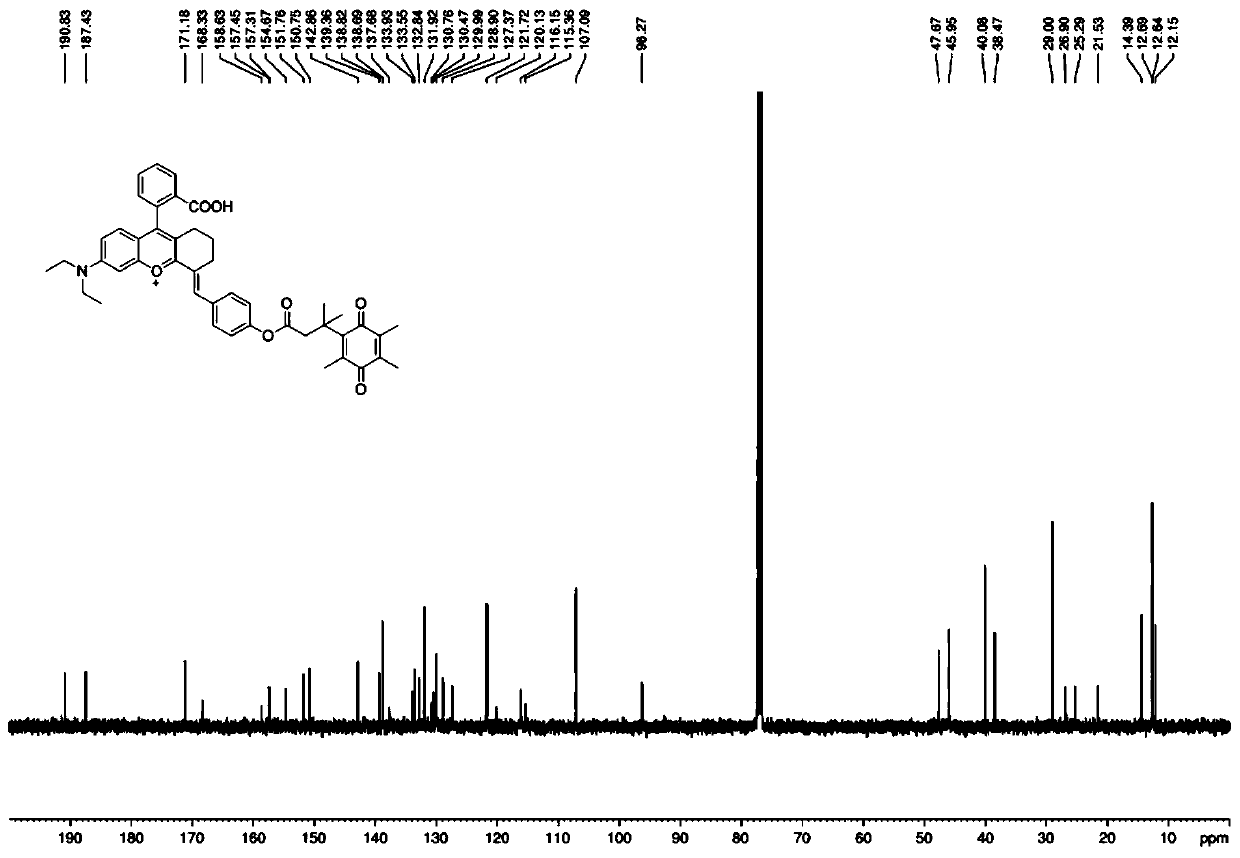
[0042] Example 1: Synthesis of fluorescent probe molecules for detection of diaphorase based on rhodamine derivatives
[0043] For specific synthetic routes, see figure 1 .
[0044] (1) Preparation of Compound 5: Under ice-bath conditions, 14 mL of concentrated sulfuric acid was added to a round-bottomed flask, and cyclohexanone (12.74 mmol, 1.32 mL) was added dropwise with a dropping funnel and stirred evenly. 2-(4-(Diethylamino)-2-hydroxybenzoyl)benzoic acid (6.4 mmol, 2.0 g) was added in portions and stirred. The mixture was heated to reflux at 90°C for 2 hours, cooled to room temperature, the reaction solution was poured into ice water, and 1.4 mL of 70% perchloric acid was added. After the precipitate was precipitated, it was suction-filtered, the filter cake was washed with water, and dried to obtain compound 5 as a black solid with a yield of 90%.
[0045] (2) Preparation of Compound 4: Dissolve Compound 5 (1.72g, 3.6mmol) in glacial acetic acid, add 4-hydroxybenzal...
Embodiment 2
[0050] Example 2: Selectivity of fluorescent probe molecules for diaphorase detection based on rhodamine derivatives
[0051] Using CH 3 OH (methanol): PBS (0.01mol / L, pH=7.4) = 9:1 (v:v) solution controls the experimental conditions.
[0052] The fluorescent probe molecule of the present invention is used CH 3 OH:PBS=9:1 (v:v) was dissolved in a solvent and fixed to a volumetric flask of 100mL, and the molecular concentration of the fluorescent probe was prepared to be 2×10 -5 mol / L solution.
[0053] The sample vials were divided into 17 groups, each group of sample vials were added with 5mL concentration of 2×10 -5 CH of the fluorescent probe molecule of the present invention of mol / L 3 OH:PBS (0.01mol / L, pH=7.4)=9:1 (v:v) solution, the first bottle of solution was used as a blank group, and 50 μL of Co at a concentration of 0.01mol / L was added to the other 16 groups 2+ , Ni 2+ ,Fe 3+ , K + ,Pb 2+ , HSO 3 - ,NO 3 - ,H 2 o 2 ,H 2 S,ClO - , Cys, Hcy, Ala, Cys...
Embodiment 3
[0054] Example 3: Fluorescence titration of diaphorase detection fluorescent probe molecules based on rhodamine derivatives to diaphorase
[0055] Using CH 3 OH (methanol): PBS (0.01mol / L, pH=7.4) = 9:1 (v:v) solution controls the experimental conditions.
[0056] Fluorescent probe molecules of the present invention are dissolved with a solvent of DMSO:PBS=9:1 (v:v) and settled in a volumetric flask of 1000mL, and the concentration of fluorescent probe molecules is prepared as 2×10 -5 mol / L solution.
[0057] 3 mg of diaphorase (DTD) was dissolved in 100 μL of deionized water to prepare an aqueous solution of diaphorase with a concentration of 900 U / mL.
[0058] Divide the sample bottles into 21 groups, add 0μL-40μL diaphorase aqueous solution with a concentration of 900U / mL to each group of sample bottles, and then use a concentration of 2×10 -5 CH of the fluorescent probe molecule of the present invention of mol / L 3 OH:PBS (0.01mol / L, pH=7.4)=9:1 (v:v) solution was adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com