Synthesis method of ferrioxamine succinimide activated ester and application thereof
A technology of ferrioxamine succinimide and oxalamine succinimide, which is applied in the field of radiopharmaceutical labeling, can solve the problems of complex and time-consuming, poor water solubility of Df-Bz-NCS, and the inability to easily measure the radiochemical purity, and achieve The effect of low cost, good stability in vivo, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1) Synthesis of compound Ⅱ-a
[0049]
[0050] Add deferoxamine methanesulfonate DFO (500mg, 0.761mmol) and succinic anhydride (760mg, 7.59mmol) into pyridine (7.5mL), shake to dissolve, and stir overnight at 25°C.
[0051] The reaction solution was added to a sodium hydroxide (125 mL, 0.06 mol / L) solution, and allowed to stand overnight at 25° C., and the pH was detected to be 7.5. Hydrochloric acid (18.5 mL, 6 mol / L) was added to the reaction solution, the pH was detected to be 2, and a white precipitate was formed. Place in a refrigerator at 4°C for 2 hours, centrifuge, wash with 0.01mol / L HCl (150mL), and dry to obtain 417mg of compound II-a as a white powder with a yield of 83%.
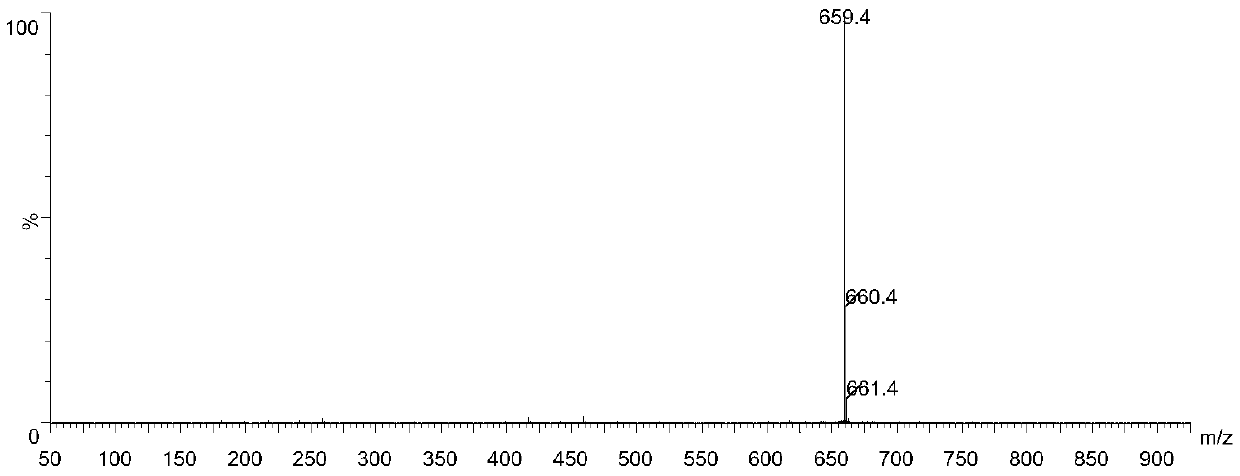
[0052] MS Spectrum: M / Z[M-H] - = 659.4.
[0053] 2) Synthesis of Compound Ⅰ-a
[0054]
[0055] FeCl 3 ·6H 2 O (17.6mg, 0.0697mmol) was dissolved in acetonitrile (1.3mL), compound II-a (20mg, 0.0303mmol) and acetonitrile (5mL) were added, shaken to dissolve, and the solution w...
Embodiment 2
[0076] The succinic anhydride used in the preparation of compound II-a in Example 1 was replaced by glutaric anhydride and adipic anhydride, respectively, to prepare the corresponding activated ester of ferrioxamine succinimide.
[0077] MS Spectrum: M / Z[M+Na] + They are 847.36 and 861.39 respectively.
Embodiment 3
[0079] The NHS of compound I-a prepared in Example 1 was replaced by DSC, 1-hydroxy-2,5-dioxo-3-pyrrolidine carboxylic acid, sodium salt of N-hydroxysuccinimide sulfonate, respectively, to prepare the corresponding Activated ester of ferrioxamine succinimide.
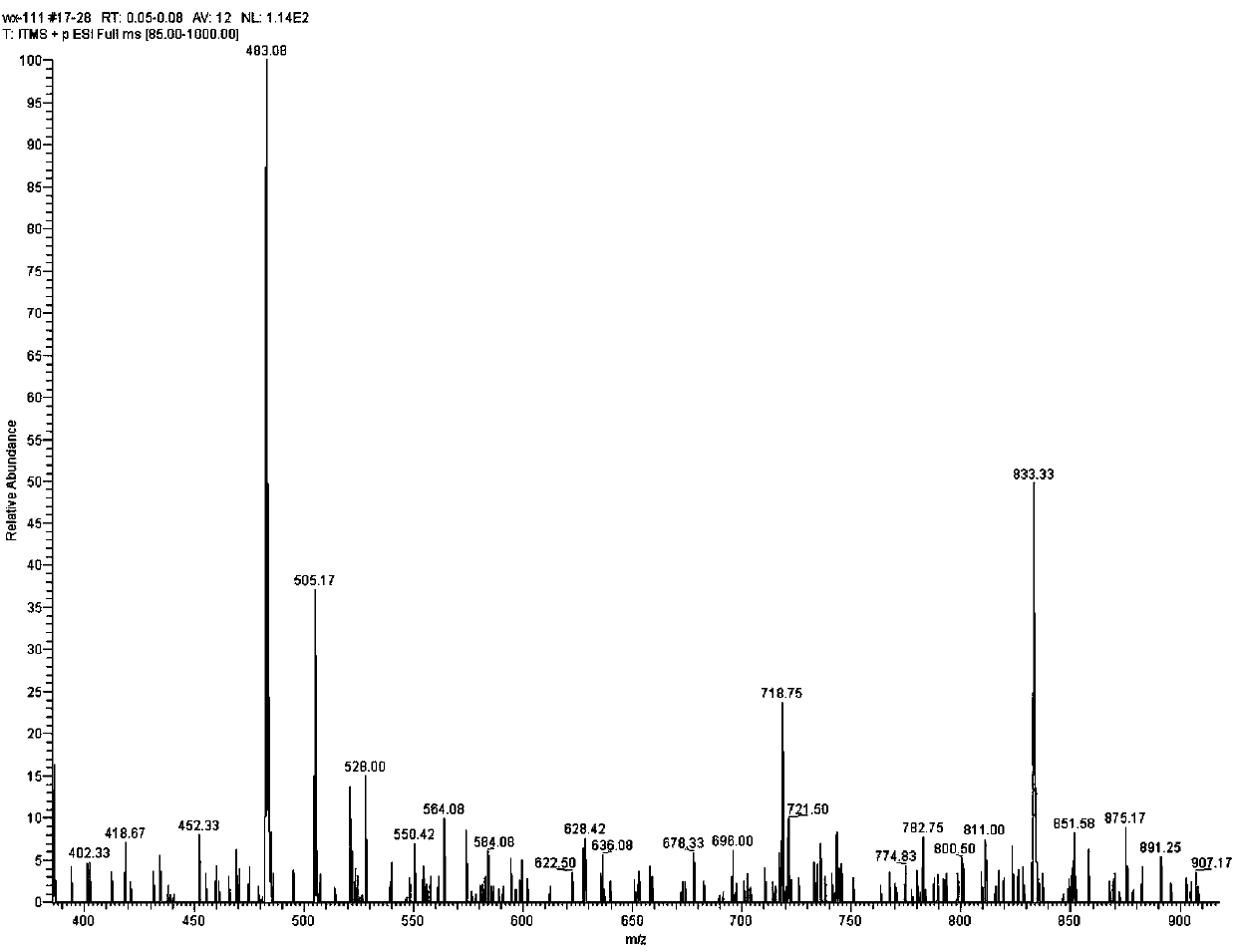
[0080] MS Spectrum: M / Z[M+Na] + They are 833.37, 877.43, 913.36 respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com