Catalyst for synthesizing dimethyl carbonate by catalytic coupling of carbon monoxide as well as preparation method and application thereof
A technology of dimethyl carbonate and carbon monoxide, which is applied in the preparation of carbonate/haloformate, preparation of organic compounds, catalyst activation/preparation, etc., can solve problems such as poor stability and inability to maintain high activity for a long time , to achieve the effects of stable reaction performance, improved electron transfer performance, high and low temperature activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
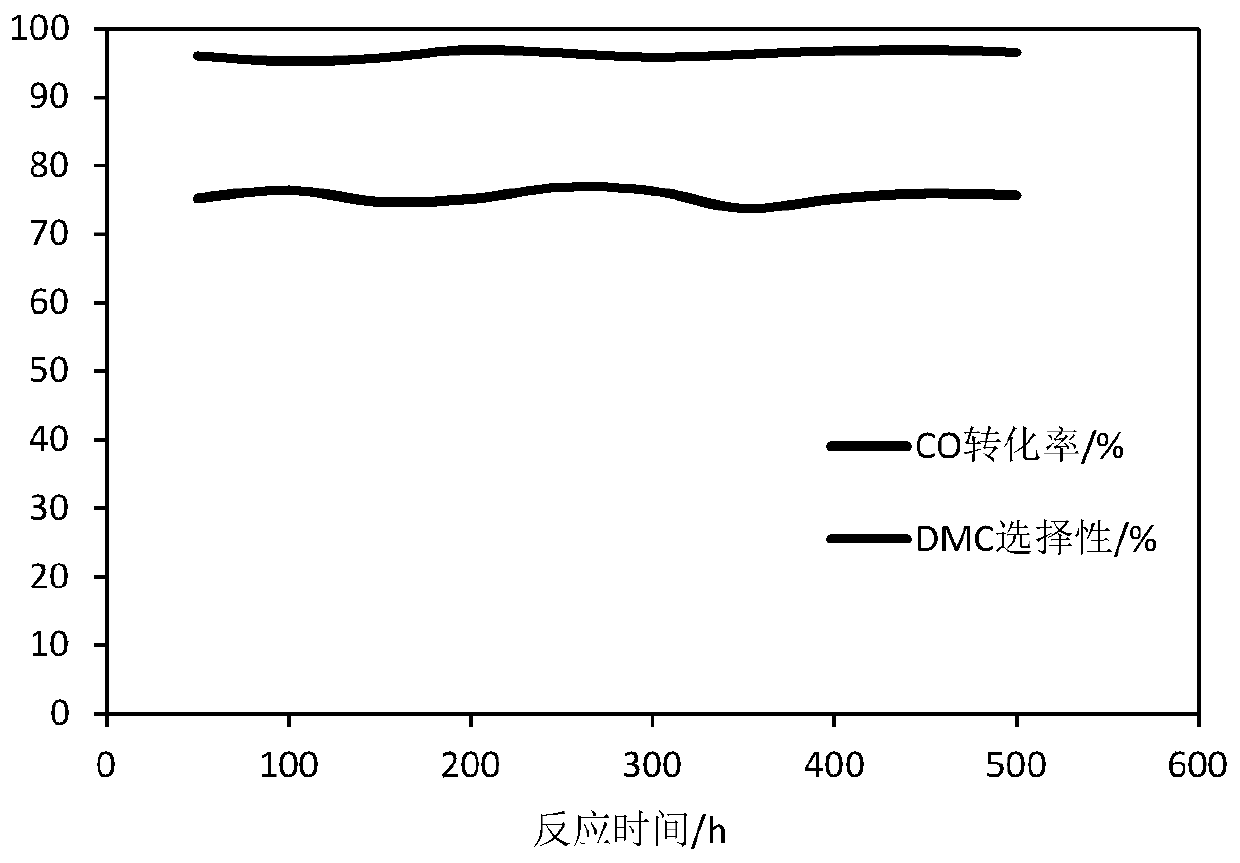
Embodiment 1
[0052] Weigh 1.35g of ammonium metatungstate and dissolve it in 83.65g of deionized water, and dissolve 100g of γ-Al 2 o 3 The carrier was added into the above solution and immersed for 60 minutes until the solution was completely absorbed by the carrier, then ultrasonically dried at 85°C for 4 hours, and calcined in a muffle furnace at 800°C for 2 hours to obtain a composite oxide carrier; weigh 1.34g of chlorine dihydrate Copper chloride was dissolved in 83.66g of deionized water, and the composite oxide carrier prepared above was immersed in the prepared copper chloride solution for 60 minutes until the solution was completely absorbed by the carrier, then ultrasonically dried at 85°C for 4 hours, and then dried at 400°C for 4 hours. Roast in a furnace for 4 hours; then weigh 0.835g of palladium chloride solid, dissolve it in 83g of aqueous solution containing hydrochloric acid, control the pH value of the solution to 1.2 to 1.3, and immerse the above-mentioned roasted samp...
Embodiment 2
[0054] Weigh 2.70g of ammonium metatungstate and dissolve it in 82.3g of deionized water, and dissolve 100g of γ-Al 2 o 3 The carrier was added into the above solution and immersed for 60 minutes until the solution was completely absorbed by the carrier, then ultrasonically dried at 85°C for 4 hours, and then roasted in a muffle furnace at 500°C for 6 hours to obtain a composite oxide carrier; weigh 5.36g of chlorine dihydrate Copper chloride was dissolved in 79.64g of deionized water, and the composite oxide carrier prepared above was immersed in the prepared copper chloride solution for 60 minutes until the solution was completely absorbed by the carrier, then ultrasonically dried at 85°C for 4 hours, and then dried at 300°C for 4 hours. Roast in a furnace for 6h; then weigh 1.67g of palladium chloride solid, dissolve it in 83.33g of hydrochloric acid-containing aqueous solution, control the pH value of the solution to 2.0, and immerse the above-mentioned roasted sample in t...
Embodiment 3
[0056] Weigh 2.7g of ammonium metatungstate and dissolve it in 82.3g of deionized water, and dissolve 100g of γ-Al 2 o 3 The carrier was added into the above solution and soaked for 60 minutes until the solution was completely absorbed by the carrier, then ultrasonically dried at 85°C for 4 hours, and calcined in a muffle furnace at 500°C for 6 hours to obtain a composite oxide carrier; weigh 7.24g of anhydrous Ferric chloride and 2.87g of potassium chloride were dissolved in 74.89g of deionized water, and the composite oxide carrier prepared above was immersed in the prepared auxiliary salt solution for 60 minutes until the solution was completely absorbed by the carrier, and then placed at 85°C Ultrasonic drying for 4 hours, roasting in a muffle furnace at 400°C for 3 hours; then weigh 2.5g of palladium chloride solid, dissolve it in 82.5g of aqueous solution containing hydrochloric acid, control the pH of the solution to 1.0, and immerse the above-mentioned roasted sample i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com