Biodegradable zwitterionic polycarbonate and application thereof
A zwitterion and polycarbonate technology, applied in the direction of drug combinations, organic active ingredients, non-central analgesics, etc., can solve the problems that hinder the long-term application of PEG derivatives, and achieve controllable molecular weight, simple preparation, and reduced The effect of the immune response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Polymerization of acrylate cyclic carbonate monomer AC (8k), the process is as follows:
[0049]
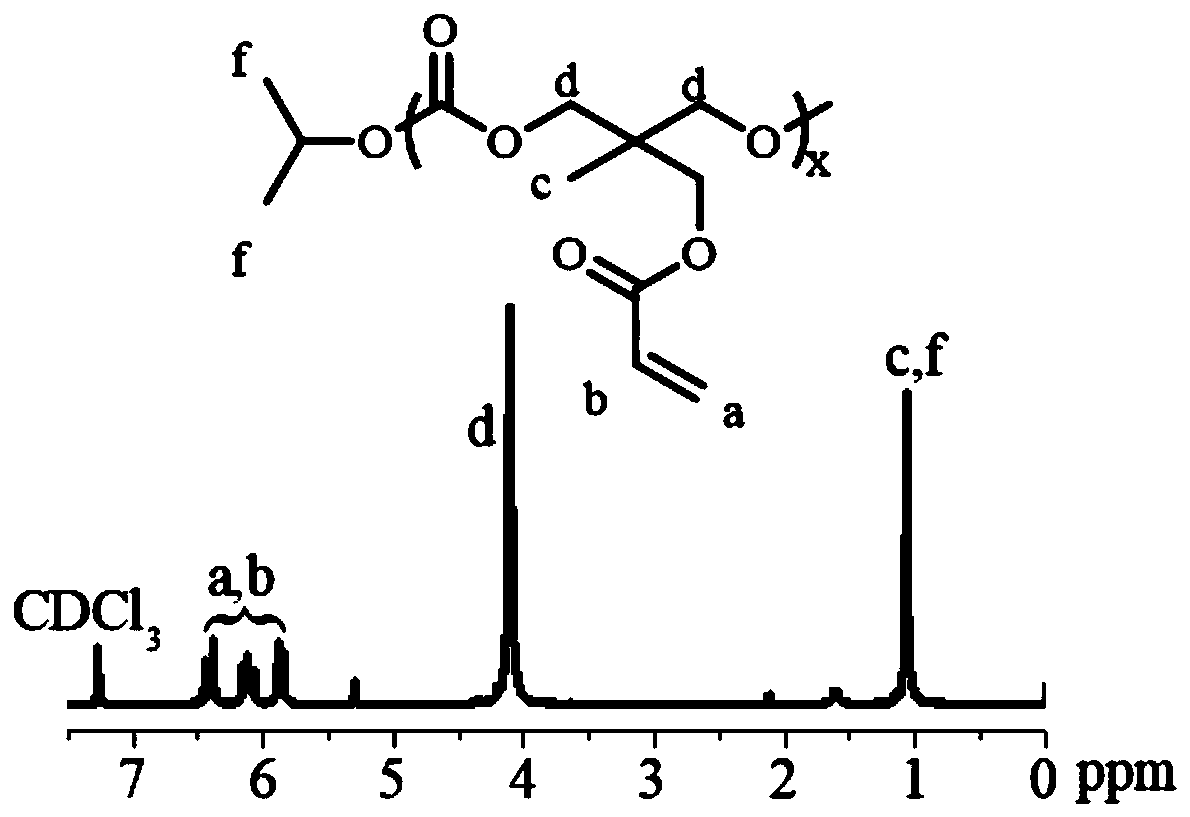
[0050] Under nitrogen protection, compound 1 (0.5g, 2.5mmol), isopropanol (0.0038g, 0.063mmol) as initiator, anhydrous dichloromethane (6mL) as solvent, bis(bistrimethylsilyl)amine zinc As a catalyst, it was carried out at 40°C and reacted for 12h. After the reaction, the polymer 1, ie, PAC, was purified by precipitation with glacial ether, and the yield was 80%. NMR characterization diagram see figure 1 shown.
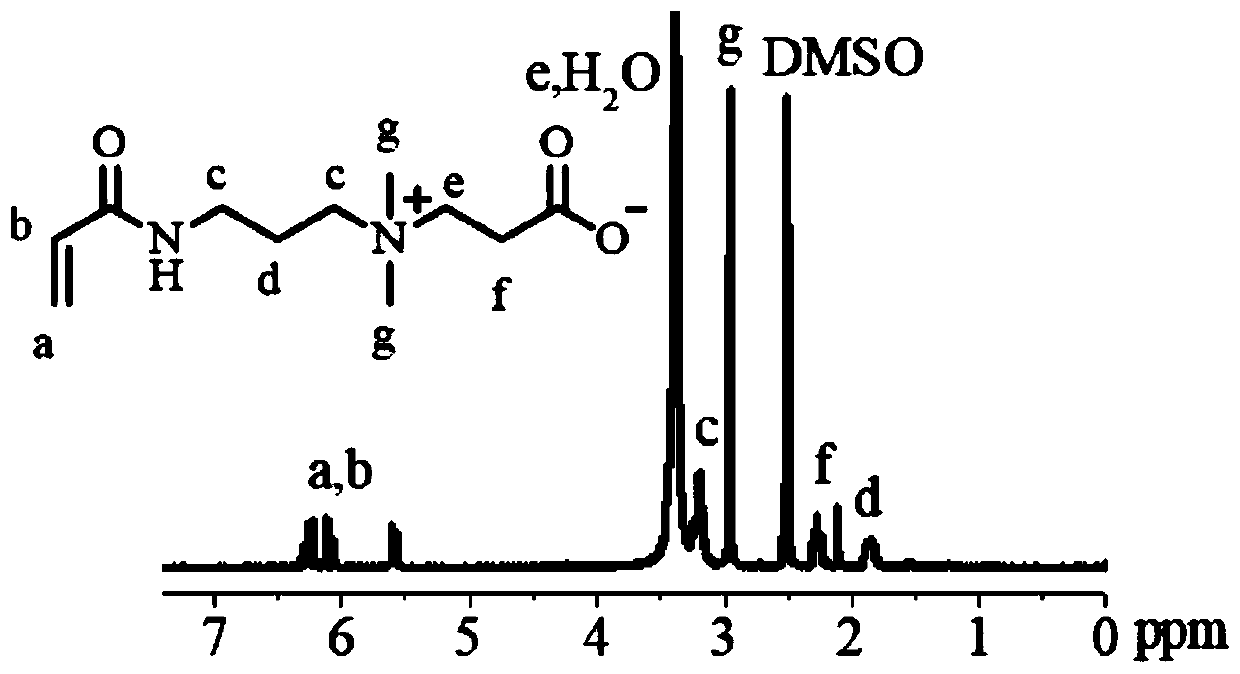
[0051] The ring-opening polymerization of polymer 1 (PAC) was carried out at 40°C with isopropanol (Mn 60) as the initiator, anhydrous dichloromethane as the solvent, and zinc bis(bistrimethylsilyl)amine as the catalyst. . The reaction was fed under nitrogen protection. After the reaction was completed, glacial acetic acid was added to terminate the reaction, and it was purified by precipitation with glacial ether. The polymer composition and GPC char...
Embodiment 2
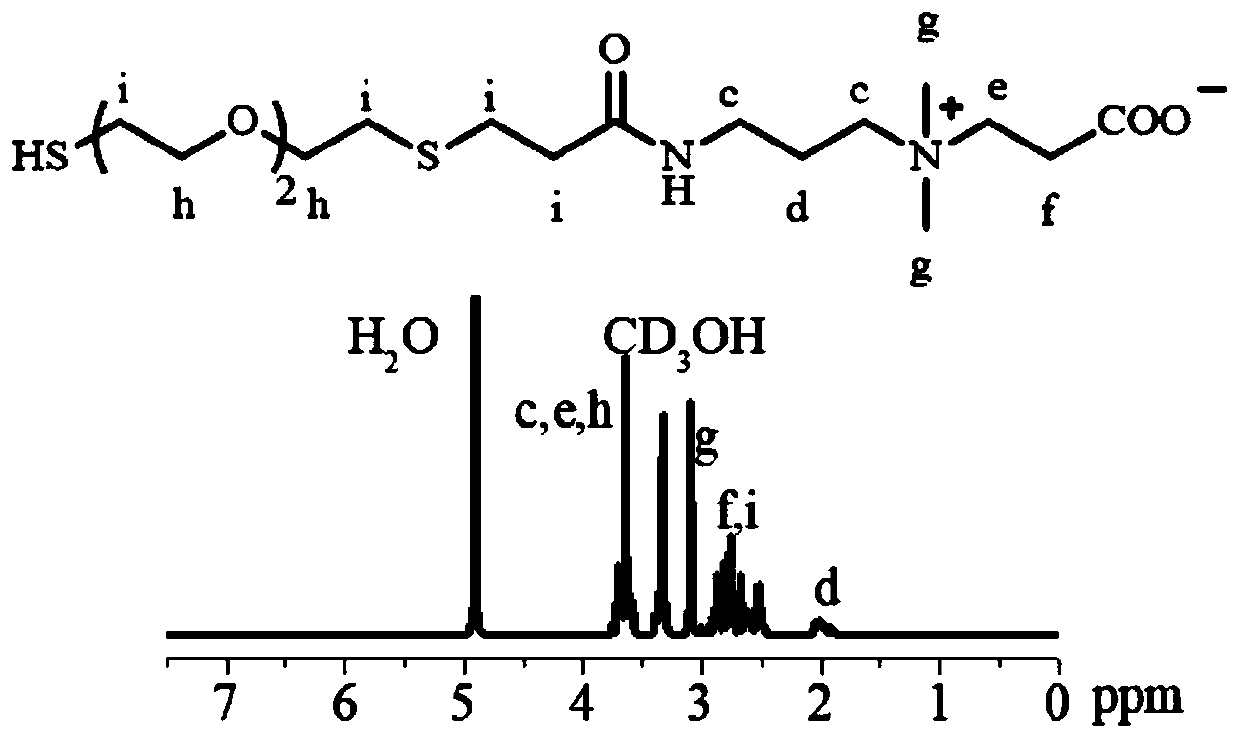
[0065] (1) Preparation of PAC(TCB)(8k) zwitterionic polymer micelles by solvent dialysis:
[0066] First, dissolve the zwitterionic polymer PAC (TCB) in a mixed solvent of N,N-dimethylformamide and methanol, put it into a dialysis bag (MWCO3500), and dialyze in a deionized water medium for 10 hours, during which the dialysis is replaced regularly medium. The average particle diameter of the micelles recorded by the dynamic light scattering instrument is 189nm, and the particle size distribution index is 0.11, such as Figure 5 shown.
[0067] (2) Changes in particle size of PAC(TCB)(8k) zwitterionic polymer micelles containing 10%wt bovine serum albumin:
[0068] Take 1mL of prepared polymer micelles with a concentration of 1mg / mL, add 10%wt bovine serum albumin to it, place it at room temperature, and measure the change of its particle size with a dynamic light scattering instrument at a specified time point.
[0069] Such as Figure 6 , under the condition of adding 10%w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com