Synthesis and application of imine spiro piperidine compounds
A compound, piperidine technology, applied in the field of medicine, can solve the problem that the new compound inhibits the antibacterial activity of chitin synthase and has not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
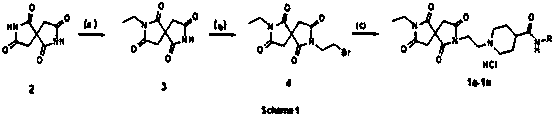
[0024] Example 1: Preparation of 2-ethyl-2,7-diazaspiro[4.4]nonane-1,3,6,8-tetraone
[0025] 2.04 g (11 mmol) of compound 2, 1.68 g (15 mmol) of bromoethane, 0.44 g (11 mmol) of triethylamine were added to a 50 mL round-bottomed flask, 20 mL of DMF was added as a solvent, and the temperature was 60°C. Under heating and stirring overnight, the solvent was removed by distillation under reduced pressure, ethyl acetate was added while hot to stir and dissolve, the solution was adjusted to acidity with dilute hydrochloric acid, the organic layer was obtained by separation, and ethyl acetate and petroleum ether were used to pass the column to obtain a white solid 1.22 g ( 5.8 mmol), yield 53%.
Embodiment 2
[0026] Example 2: Preparation of 2-(2-bromoethyl)-7-ethyl-2,7-diazaspiro[4.4]nonane-1,3,6,8-tetraone
[0027] 5.13 g (24.4 mmol) of compound 3, 1.17 g (29.3 mmol) of sodium hydroxide, 7.3 g (39 mmol) of 1,2-dibromoethane and 30 mL of DMF were added to a 100 mL round-bottomed flask, and the mixture was heated at 80 °C. Under the conditions of heating and stirring overnight, the solvent was distilled off under reduced pressure, water and ethyl acetate were added while hot, the organic layer was obtained by liquid separation, and ethyl acetate and petroleum ether were used to pass through the column to obtain 4.48 g (14 mmol) of yellow solids in a yield of 58 %. 1H NMR (600 MHz, CDCl3) δ 4.06–3.89 (m, 2H), 3.61 (dd, J = 14.2, 7.1 Hz, 2H), 3.57 (t, J= 6.5 Hz, 2H), 3.37–3.24 (m, 2H), 2.84–2.71 (m, 2H), 1.20 (t, J= 7.2 Hz, 3H).
Embodiment 3
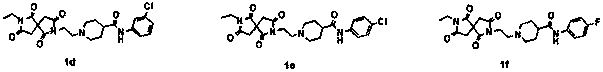
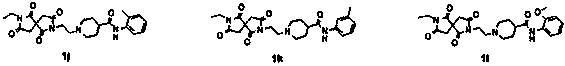
[0028] Example 3: N-(3-Bromophenyl)-1-(2-(7-ethyl-1,3,6,8-tetraoxo-2,7-diazaspiro[4.4]nonane- 2-yl)ethyl)piperidine-4-carboxamide hydrochloride
[0029]In a 25 mL flask, 0.254 g (0.8 mmol) of intermediate compound 4, 0.272 g (0.96 mmol) of intermediate 4-piperidinecarboxylic acid derivative (N-(3-bromophenyl) piperidine-4-carboxamide) were added ), 0.132 g (0.96 mmol) of ground potassium carbonate, a catalytic amount of KI, 3 mL of acetonitrile, heated and stirred at 70 °C for 4 h, filtered, and passed through a column with ethyl acetate to obtain 0.24 g of a viscous liquid, After the liquid was acidified with concentrated hydrochloric acid, the liquid was removed and recrystallized to obtain 0.14 g of white crystals with a yield of 25%. 1H NMR (600 MHz, DMSO-d6) δ 10.91 (d, J= 152.1 Hz, 1H), 10.34 (d, J = 7.3 Hz, 1H), 7.99 (d, J= 27.8 Hz, 1H), 7.53 (dt , J= 11.9, 5.9 Hz, 1H), 7.33–7.17 (m, 2H), 3.95–3.80 (m, 2H), 3.69–3.40 (m, 4H), 3.35–2.91 (m, 8H), 2.72 (d , J=120.6 Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com