Synthesis method of sex pheromone (S)-14-methyl-1-octadecene of lepidoptera pest peach leaf miners
A technology of octadecene and synthesis method, which is applied in the field of synthesis of sex pheromone-14-methyl-1-octadecene of the Lepidoptera pest Peach leafminer, which can solve the problem of expensive raw materials and long steps question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
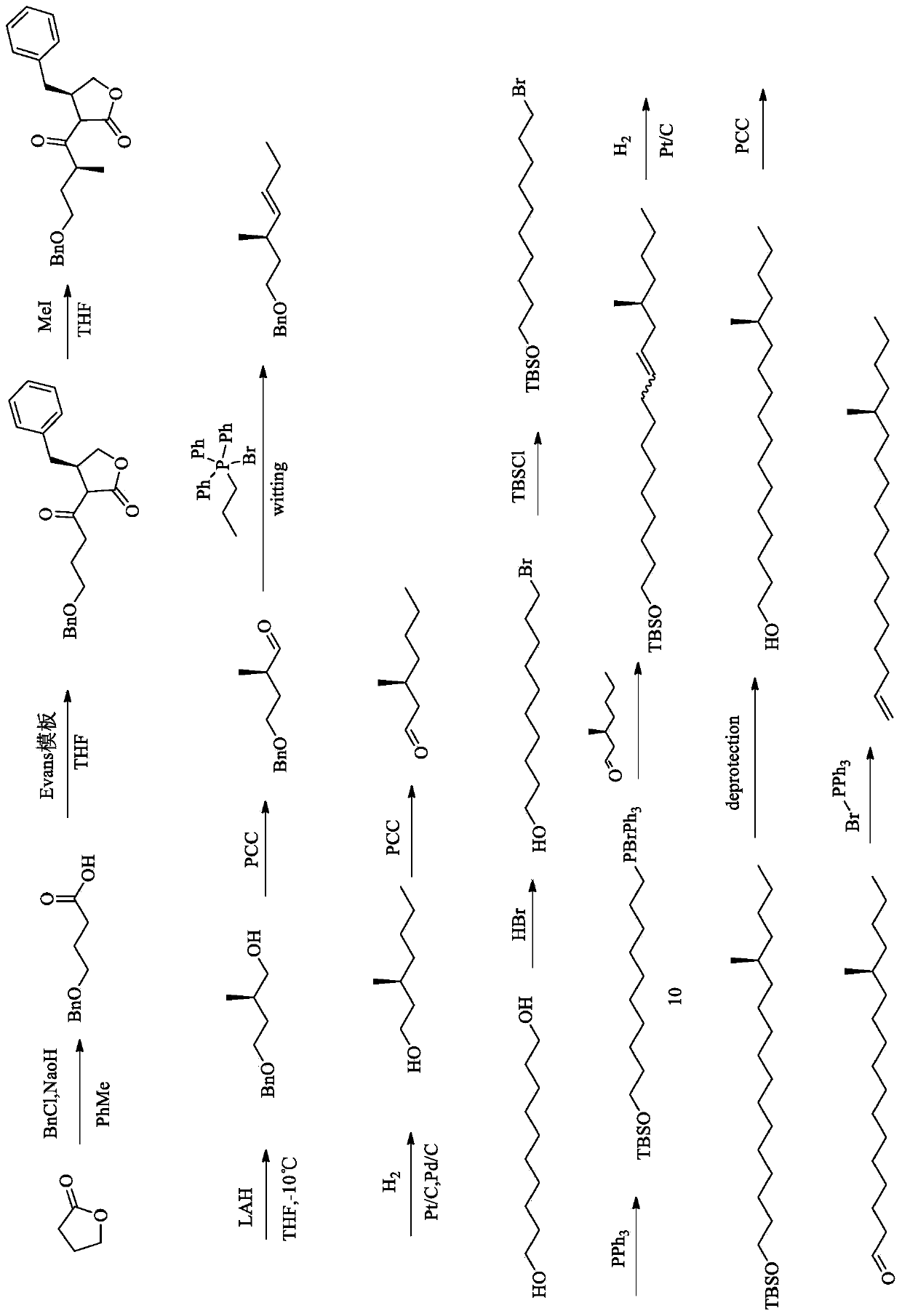
[0048] The synthesis method of the sex pheromone (S)-14-methyl-1-octadecene of the Lepidoptera pest Peach leafminer in this embodiment specifically includes the following steps (synthetic route is as follows: figure 1 shown).
[0049] Step (1) gamma-butyrolactone makes 4-benzyloxybutyric acid
[0050] Take a 500mL three-necked flask and add γ-butyrolactone (50g, 580.79mmol, 1eq), toluene 250mL, NaOH (104.54g, 2.61mol, 5eq), and benzyl chloride (294.08g, 2.32mol, 4eq) dropwise. Heating to reflux for 48h. Gas chromatography GC detects that there is no raw material point, 500mL water liquid, and retains the water phase. The aqueous phase was extracted with dichloromethane (200mL×3, that is, extracted with 200mL dichloromethane each time, a total of 3 extractions), the pH of the aqueous phase was adjusted to 2-3 under ice cooling, and the aqueous phase was extracted with ethyl acetate (200mL ×3, that is, each extraction with 200 mL of ethyl acetate, a total of 3 extractions), t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com