Method for synthesizing oxiracetam by adopting NaA type molecular film reactor
A membrane reactor and reaction technology, applied in the field of biomedicine, can solve the problems of low product yield, inability to reuse a large amount of water-containing ethanol, high separation energy consumption, and achieve the effects of high product yield, excellent dehydration performance and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
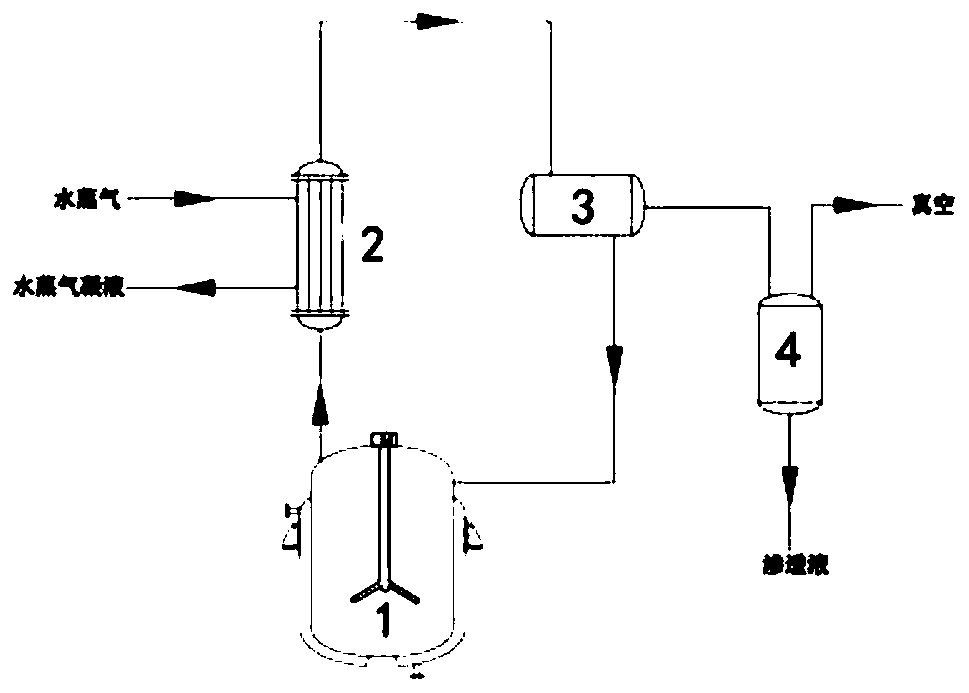
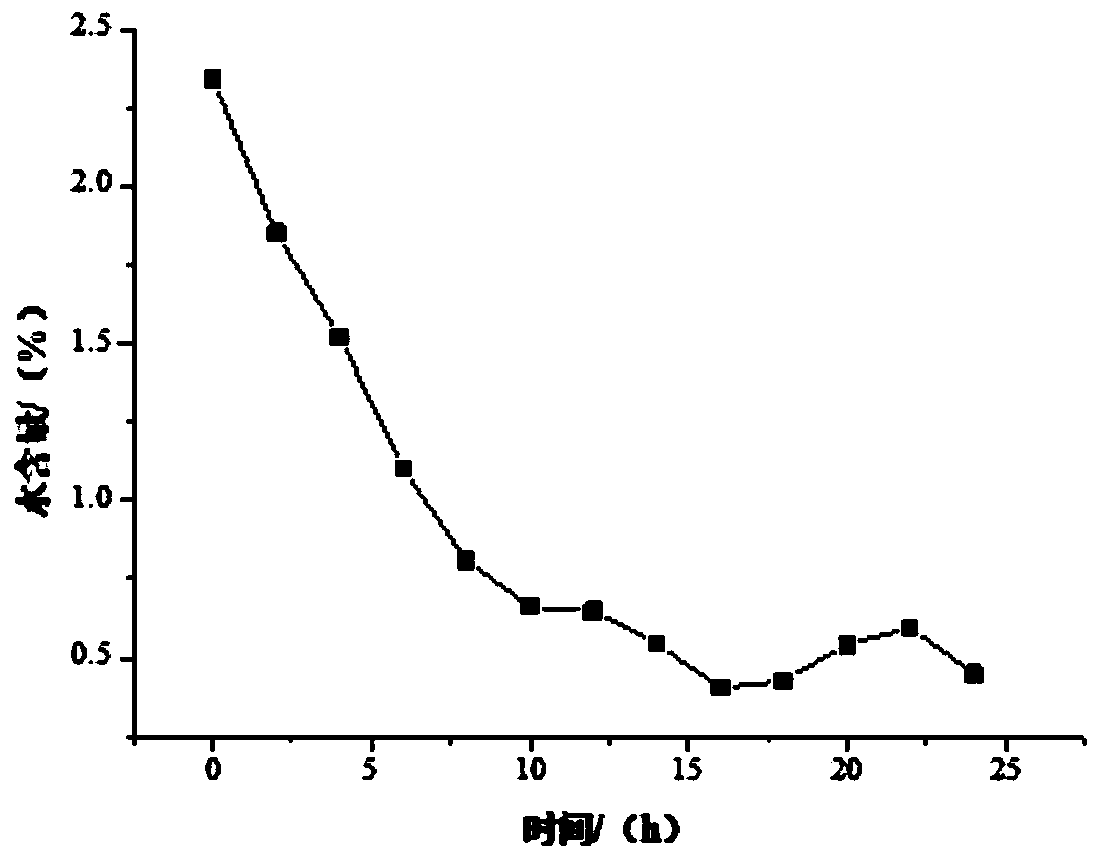
[0031] Add 480 g of absolute ethanol to the reaction flask, add 55 g of glycine hydrochloride and 85 g of sodium bicarbonate under stirring, and heat up to reflux; start to drop 90 g of 4-chloro-3-hydroxybutyrate into the reaction solution, Continue to reflux and stir; when the reflux is stable, place the NaA-type molecular sieve membrane reactor above the reaction liquid, couple with the ring-closure reaction device and turn on the vacuum, the vacuum degree is 0.05Mpa, the reaction temperature and reaction time are 80°C and 24h respectively , the ratio of membrane area to reaction solution volume is 1cm 2 / cm 3 . During the reaction process, the ethanol reflux liquid was collected every 2 hours, and its water content was detected by a Karl Fischer moisture analyzer. After the cyclization reaction was completed, the reaction product was detected by high-performance liquid chromatography, and the product yield was analyzed and calculated. The water content of ethanol is shown...
Embodiment 2
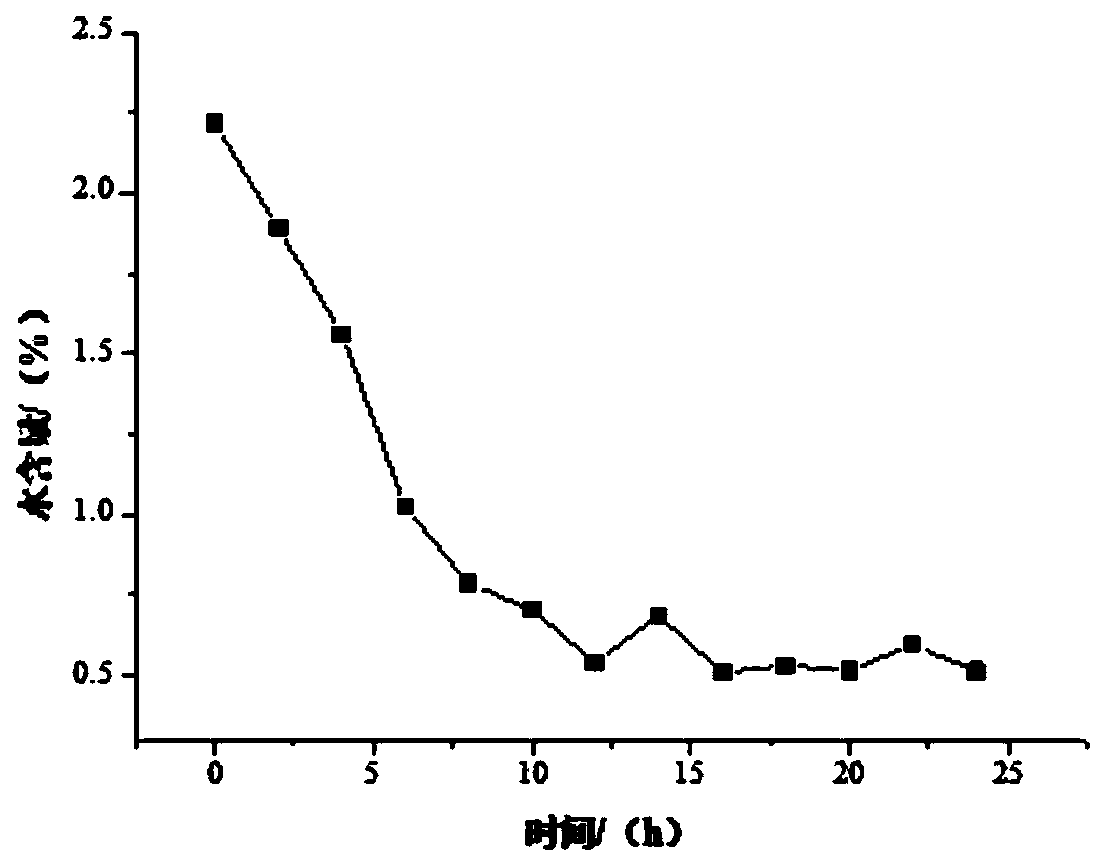
[0033] Add 500 g of absolute ethanol to the reaction flask, add 60 g of glycine hydrochloride and 90 g of sodium bicarbonate under stirring, and heat up to reflux; start to drop 95 g of 4-chloro-3-hydroxybutyrate into the reaction solution, Continue to reflux and stir; when the reflux is stable, place the NaA-type molecular sieve membrane reactor above the reaction liquid, couple with the ring-closure reaction device and turn on the vacuum, the vacuum degree is 0.05Mpa, the reaction temperature and reaction time are 85°C and 24h respectively , the ratio of membrane area to reaction solution volume is 1cm 2 / cm 3 . During the reaction process, the ethanol reflux liquid was collected every 2 hours, and its water content was detected by a Karl Fischer moisture analyzer. After the cyclization reaction, the reaction product was detected by high-performance liquid chromatography, and the product yield was analyzed and calculated. The water content of ethanol is shown in the accomp...
Embodiment 3
[0035] Add 500 g of absolute ethanol to the reaction flask, add 55 g of glycine hydrochloride and 85 g of sodium bicarbonate under stirring, and heat up to reflux; start to drop 90 g of 4-chloro-3-hydroxybutyrate into the reaction solution, Continue to reflux and stir; when the reflux is stable, place the NaA-type molecular sieve membrane reactor above the reaction liquid, couple with the ring-closure reaction device and turn on the vacuum, the vacuum degree is 0.05Mpa, the reaction temperature and reaction time are 85°C and 24h respectively , the ratio of membrane area to reaction solution volume is 1cm 2 / cm 3 . During the reaction process, the ethanol reflux liquid was collected every 2 hours, and its water content was detected by a Karl Fischer moisture analyzer. After the cyclization reaction, the reaction product was detected by high-performance liquid chromatography, and the product yield was analyzed and calculated. The water content of ethanol is shown in the accomp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com