Human REV3L protein cleavage inhibitor and application thereof
A technology of inhibitors and active inhibitors, which can be used in peptide/protein components, animal/human peptides, DNA/RNA fragments, etc., and can solve problems such as tumor recurrence and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Confirmation of post-translational cleavage-dependent sequence elements
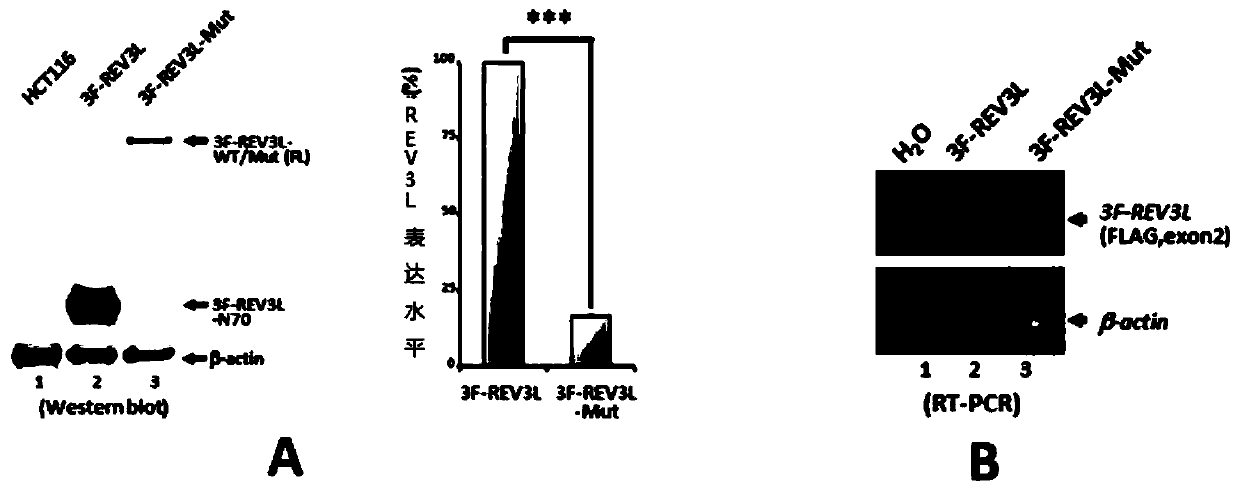
[0034] After inserting a FLAG tag coding sequence in the 5' upstream of the endogenous REV3L gene coding region in human HCT116 cells, it was found that a protein with a FLAG tag of about 70 kDa was expressed in the cells. After identification by anti-FLAG immunopurification coupled to mass spectrometry under denaturing conditions, it was finally confirmed that the 70 kDa protein with FLAG tag was REV3L protein with C-terminal deletion.
[0035] Using transient expression of a series of REV3L deletion mutants with N-terminal and C-terminal fusions of FLAG and HA tags in HEK293 cells, it was found that the 70kDa C-terminal deletion REV3L protein was generated by a site-specific post-translational protein cleavage event , and the cleavage product was named REV3L-N70, and its amino acid sequence is shown in SEQ ID No.5.
[0036] Through further expression experiments of deletion mutants an...
Embodiment 2
[0037] Example 2 Verifies the relationship between sequence elements, protease TASP1 and human REV3L protein
[0038] In human HEK293 cells expressing the REV3L N-terminal fragment containing the QLDGTAD cleavage sequence, overexpression of wild-type TASP1 can significantly increase the protein cleavage efficiency of the REV3L N-terminal fragment. At the same time, overexpression of TASP1-T234A mutant (TASP1-Mut) without protease activity has no corresponding effect.
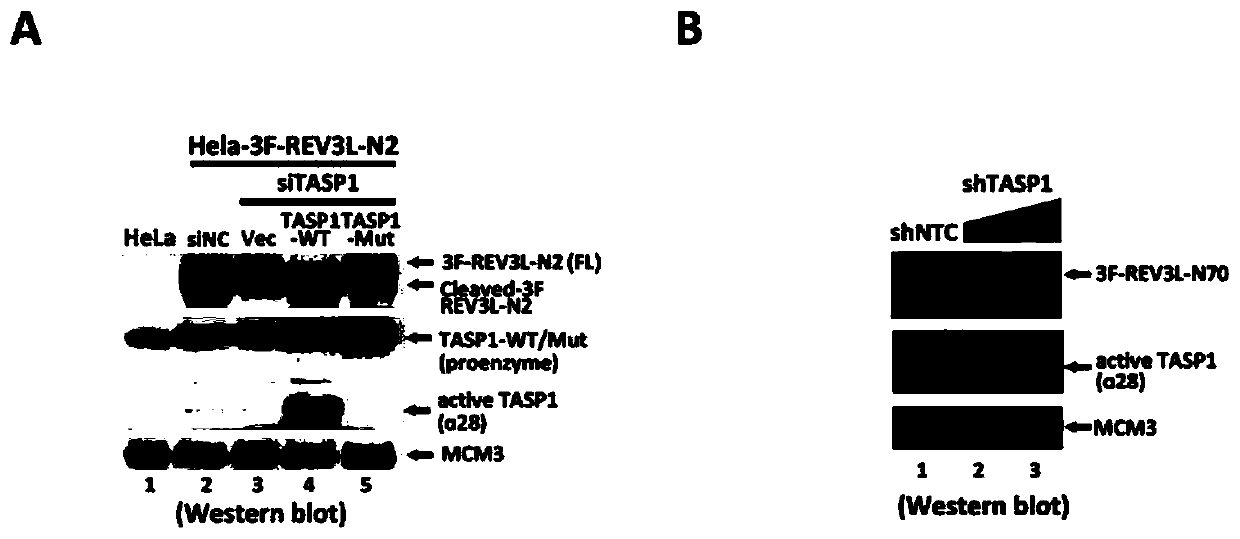
[0039] A stable cell line expressing the N-terminal fragment of REV3L containing the QLDGTAD cleavage sequence was constructed in HeLa cells. In this cell line, TASP1 expression was reduced by siRNA-mediated RNA interference. The result is as figure 1 As shown in A, cleavage of the REV3L N-terminal fragment was significantly inhibited.
[0040] ShRNA-mediated RNA interference reduces TASP1 expression in human colon cancer-derived HCT116 cells. The result is as figure 1 As shown in middle B, the cleavage pro...
Embodiment 3
[0042] Example 3 Effect of post-translational sequence-dependent cleavage of REV3L by TASP1 protease on REV3L protein stability and DNA polymerase ζDNA synthesis activity
[0043] Using the CRISPR / Cas9-mediated homologous recombination strategy, a site-directed mutation was introduced into the endogenous REV3L gene of human rectal cancer cell HCT116, resulting in alanine substitution in the cleavage-dependent sequence element of the REV3L mutant encoded by it, resulting in expression cleavage Mutant cell lines deficient in the REV3L-Mut protein. The changes in the REV3L gene sequence in the mutant cells were verified by genomic DNA sequencing. The effect of mutation on REV3L protein cleavage was verified by Western blot.
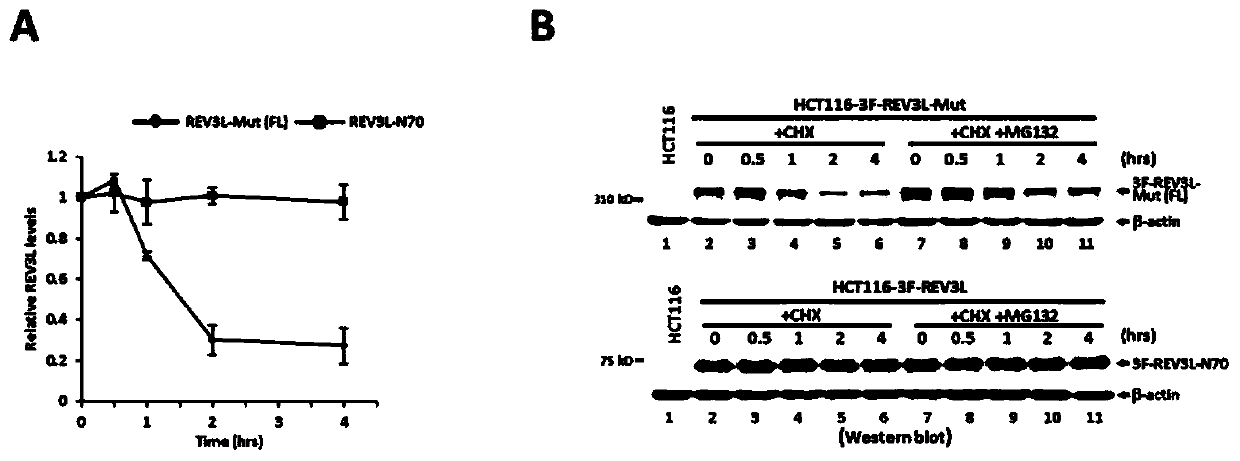
[0044] 1. Uncleaved full-length human REV3L protein can undergo proteasome-mediated protein degradation through K48-polyubiquitination
[0045] a) Comparison of cleavage-deficient REV3L-Mut mutant cells and wild-type cells, such as figure 2 As shown in A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com