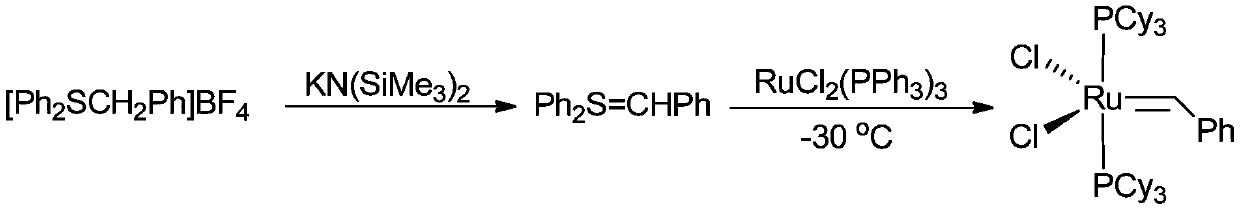Synthesis method of ruthenium carbene catalyst
A carbene catalyst and a synthesis method technology are applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., and can solve the problems of complicated post-processing and unfavorable industrial production, etc. Achieve the effect of easy availability of raw materials, convenient raw materials and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
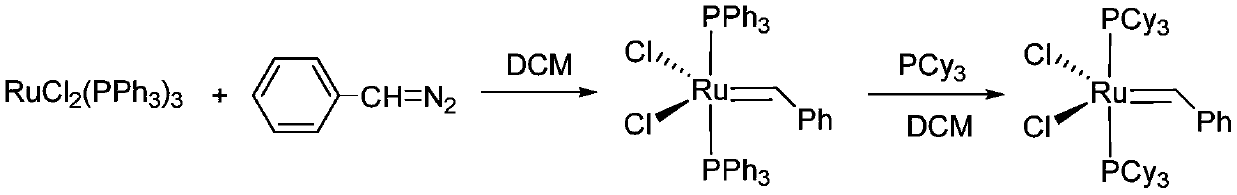
[0043] Embodiment 1: the synthesis of Grubbs catalyst
[0044] Step 1)C 6 h 5 CH=PPh 3 preparation of
[0045] Add 20.7g triphenylphosphine (PPh 3 ) and 50ml of toluene, stirred at 40°C for 0.5 hours until triphenylphosphine was completely dissolved, slowly added 6.8g of benzyl bromide (C 6 h 5 CH 2 Br), keep a constant temperature of 40°C for 4 hours, the reaction solution gradually becomes cloudy, and it is observed that a large amount of white solids are formed in the reaction system. Suction filtration, washing obtains white solid [C 6 h 5 CH 2 -PPh 3 ]Br about 16.5g, yield 96%. 1 H NMR (C 6 D. 6 ,500M):δ7.63-7.70(m,6H),7.01-7.71(m,7H),6.92-6.96(m,6H),6.67-6.69(m,1H),2.85(d,1H),; 31 P NMR (C 6 D. 6 ,200M):δ7.9(s,PCy 3 ).
[0046] Get 100ml Schlenk reaction bottle and add 4.32g [C 6 h 5 CH 2 -PPh 3 ]Br, replace the air 3 times, inject 40ml of anhydrous ether, inject 7.5ml of n-butyllithium-n-hexane solution (1.6M) into the above system under the condit...
Embodiment 2
[0054] Compared with Example 1, most of them are the same, and the benzyl bromide in step 2) is replaced with benzyl chloride, and white solid [C 6 h 5 CH 2 -PPh 3 ]Cl about 10.3g, yield 67%. 1 H NMR (C 6 D. 6 ,500M):δ7.69-7.75(m,9H),7.58-7.61(m,6H),7.18-7.20(m,1H),7.07-7.10(m,4H),5.45(d,2H),; 31 P NMR (C 6 D. 6 ,200M):δ23.6(s,PCy 3 ).
[0055] Get 100ml Schlenk reaction bottle and add 3.88g [C 6 h 5 CH 2 -PPh 3 ]Cl, replace the air 3 times, inject 40ml of anhydrous ether, inject 7.5ml of n-butyllithium-n-hexane solution (1.6M) into the above system under ice-water bath conditions, stir at room temperature for 2 hours, and the reaction solution gradually becomes orange-red The reaction was stopped, and the orange-red liquid was obtained by vacuum filtration, which was C 6 h 5 CH=PPh 3 , the reaction solution can be directly put into the next reaction. 31 P NMR (C 6 D. 6 ,200M):δ9.7(s,PCy 3 ).
[0056] Further complexation reaction with triphenylphosphine r...
Embodiment 3
[0058] Compared with Example 1, most of them are the same, and the reaction medium dichloromethane in step 2) is replaced with methyl tetrahydrofuran.
[0059] Take another 100ml Schlenk reaction bottle, and add 4.5g RuCl to the glove box 2 (PPh 3 ) 3 , add 50ml of dry methyl tetrahydrofuran under nitrogen atmosphere, and slowly add the C prepared in step (1) under the condition of -30°C 6 h 5 CH=PPh 3 (2 times equivalent, in [C 6 h 5 CH 2 -PPh 3 ] X meter, X=Cl or Br), the color of the reaction solution gradually deepens, and 5.6gPCy is added after stirring for 2 hours 3 , return to room temperature and stir for 1 hour, and the reaction ends. The reaction solvent was removed under vacuum, recrystallized, washed, and filtered to obtain Grubbs I catalyst, 1.7 g, yield 41%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com