Application of jzy-17 and compound in preparation of medicament for treating psoriasis
A technology of JZY-17 and compound, which is applied in the field of medicaments for the treatment of psoriasis, can solve the problems of few and insufficient keratinocytes, and achieve the effect of good market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1 Isolation and cultivation of keratinocytes
[0022] The foreskin tissue after male circumcision was taken, and put into PBS with gentamicin and amphotericin immediately after the operation, and the tissue was washed 3 times with PBS to remove blood stains. Prepare the keratinocyte culture medium with HKGS, gentamicin / amphotericin (500X) in advance, put the tissue into the culture medium, remove the subcutaneous tissue, cut the skin tissue into small pieces, about 5mmX5mm, add dispaseII Enzyme (1.2U / ml) was kept away from light at 4°C overnight; the epidermis and dermis were separated, and the epidermis was digested with 0.5% trypsin at 37°C for about 5-10min, and the digestion was terminated with DMEM medium containing 10% serum at 200 mesh Grind, filter, centrifuge on a strainer, wash twice with PBS, resuspend the cells in the epidermal cell culture medium added with antibiotics, and plate; 3-4 days, the cells can adhere to the wall, and the fibroblasts do no...
Embodiment 2
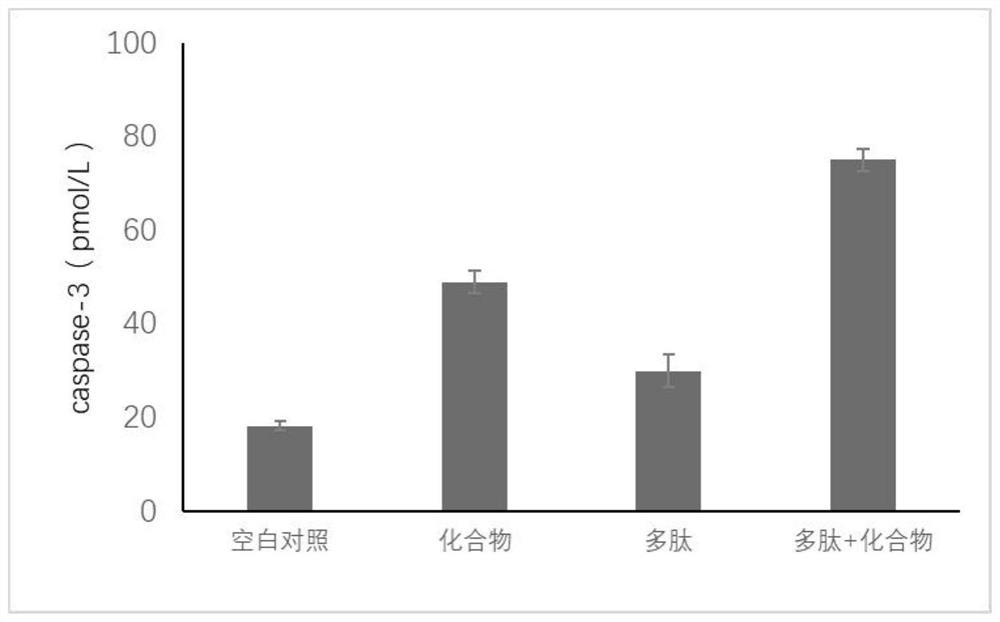
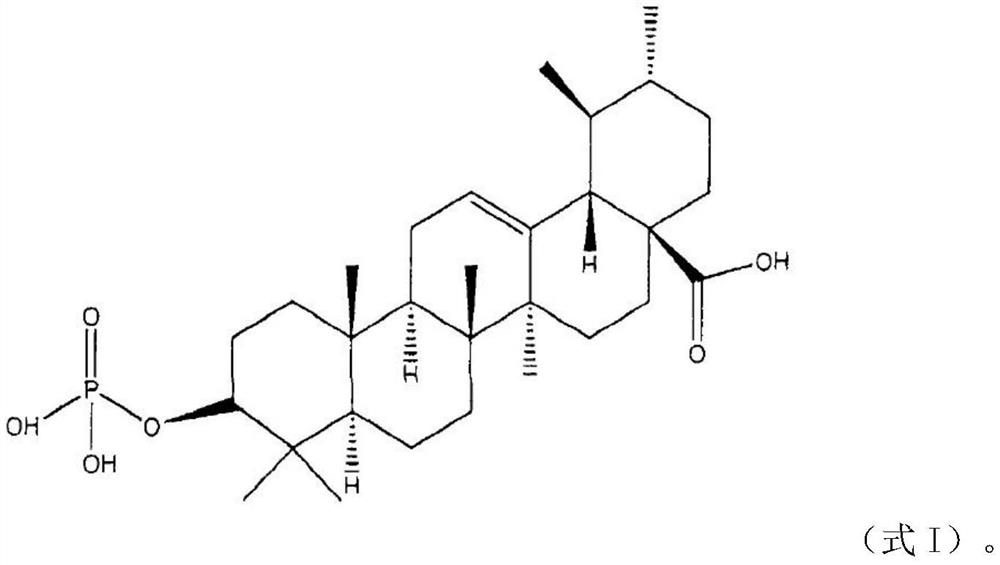
[0023] The preparation of embodiment 2 keratinocyte inhibitory compounds
[0024] A mixture of ursolic acid (48.1 g, 0.105 mol), dimethyl-N, N-diethyl phosphoramidate (34.82 g, 0.211 mol), and dry tetrahydrofuran (1250 ml) was heated to 35° C. to prepare a transparent solution, and 1H-tetrazole (44.25 g, 0.632 mol) was added in one portion at an internal temperature of 27°C, followed by stirring at room temperature (22°C) for 1 hour. After production of dimethyl phosphite was confirmed by TLC, the reaction solution was cooled with acetone-dry ice, and a 70% aqueous solution of tert-butyl hydroperoxide (84 mL, 0.607 mol) was added dropwise thereto at -20°C. The ice bath was removed, the temperature was gradually raised to room temperature, TLC was performed to confirm the disappearance of dimethyl phosphite and the production of dimethyl phosphate, and then the reaction was terminated with 10% aqueous sodium bisulfite (300 ml) at 0°C. Ethyl acetate (1250 mL) was added to the r...
Embodiment 3
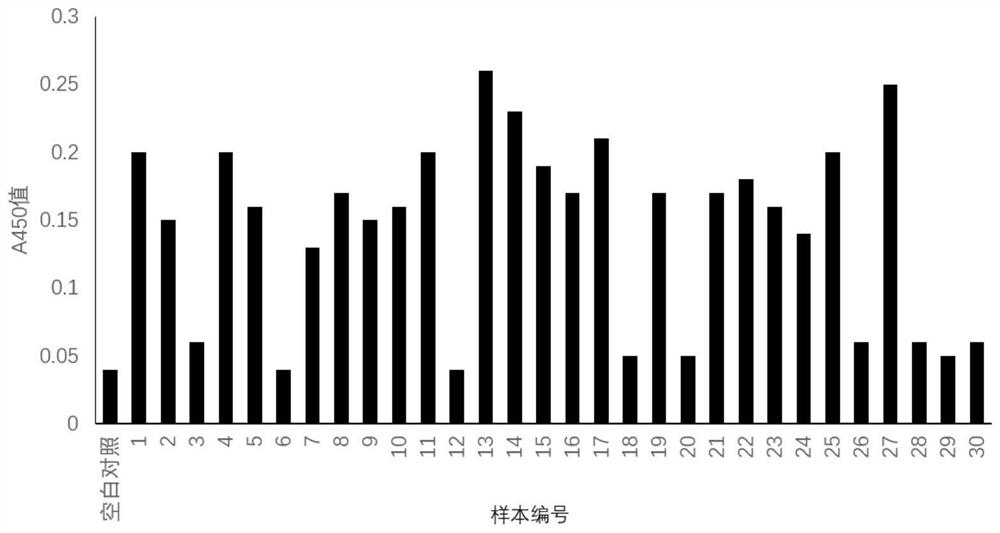
[0025] Example 3 Keratinocyte Inhibitor Polypeptide Screening
[0026]Phage Random 12 Peptide Library Kit (Ph.D.TM-12 Phage Display Peptide Library Kit) was purchased from New England Biolabs, USA, and the host bacteria was E.coliER2738.
[0027] Random dodecapeptide phage display library biological screening: use 0.1mol / L polylysine to carry out the pretreatment of microwell plate, 1) coating: use the keratinocyte 1 * 10 that embodiment 1 prepares 6 pcs / mL coated microwell plate, put in a humid box at 4°C overnight, then add 200 μl / well of fixative (glutaraldehyde) and wash 3 times; 2) Binding: blocking buffer [0.1mol / L NaHCO 3 (pH8.6), 5mg / mL BSA, 0.02% NaN 3 〕Seal, wash the plate 6 times with TBST, dilute 10 μL of the original library with 90 μL of TBST and add it to the microwell, shake gently at room temperature for 60 min. 3) Elution: Pour off the liquid, wash the plate 10 times, add 100 μL / well of 0.2mol / L glycine-hydrochloric acid buffer (pH2.2), shake at room temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com