Hydroxycamptothecine linoleate micromolecular prodrug and construction of self-assembled nanoparticles
A technology of hydroxycamptothecin linoleate and self-assembled nanoparticles, which is applied in the direction of drug combination, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of no obvious advantages and poor clinical results Ideal and other problems, to achieve the effect of simple and easy preparation method, simple and easy synthesis method, and high-efficiency entrapment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
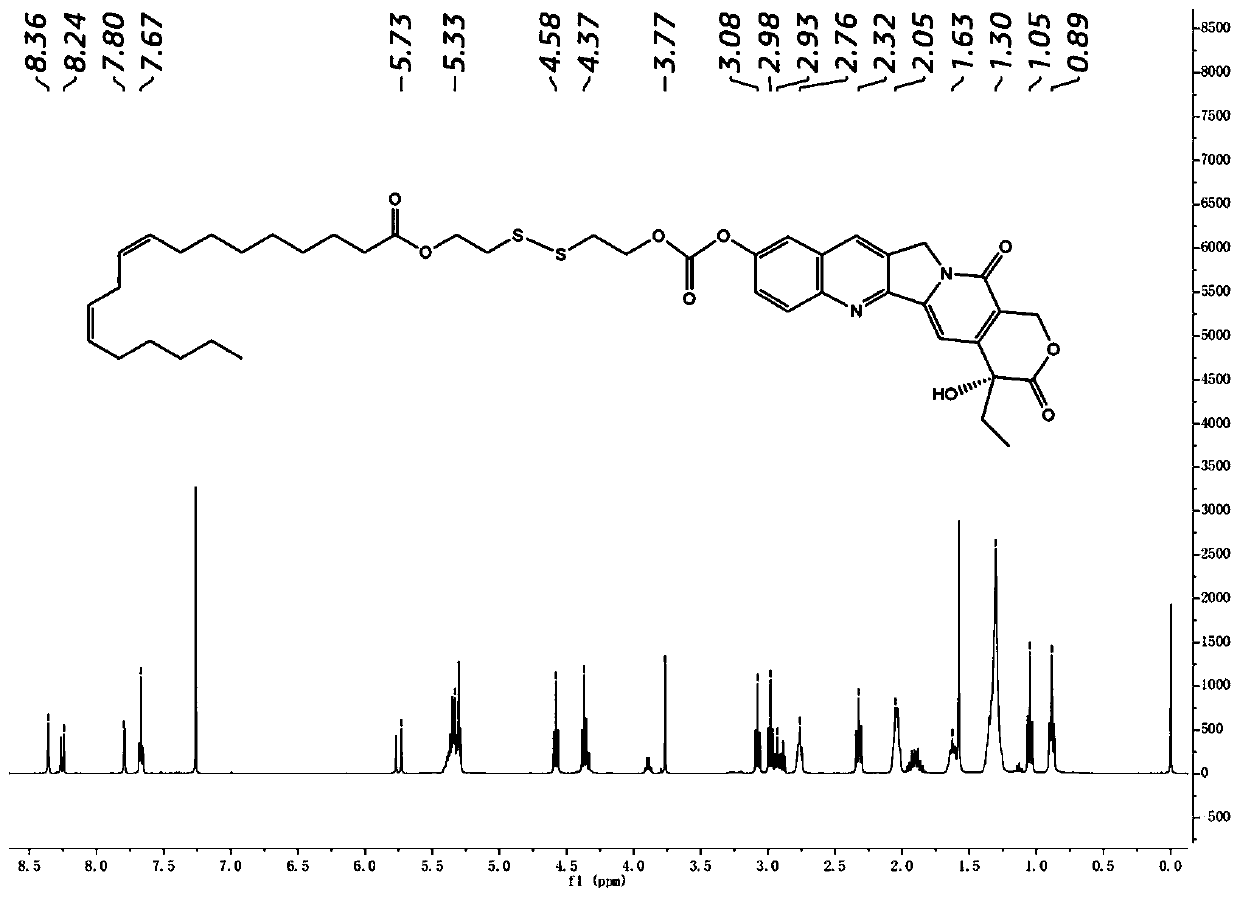
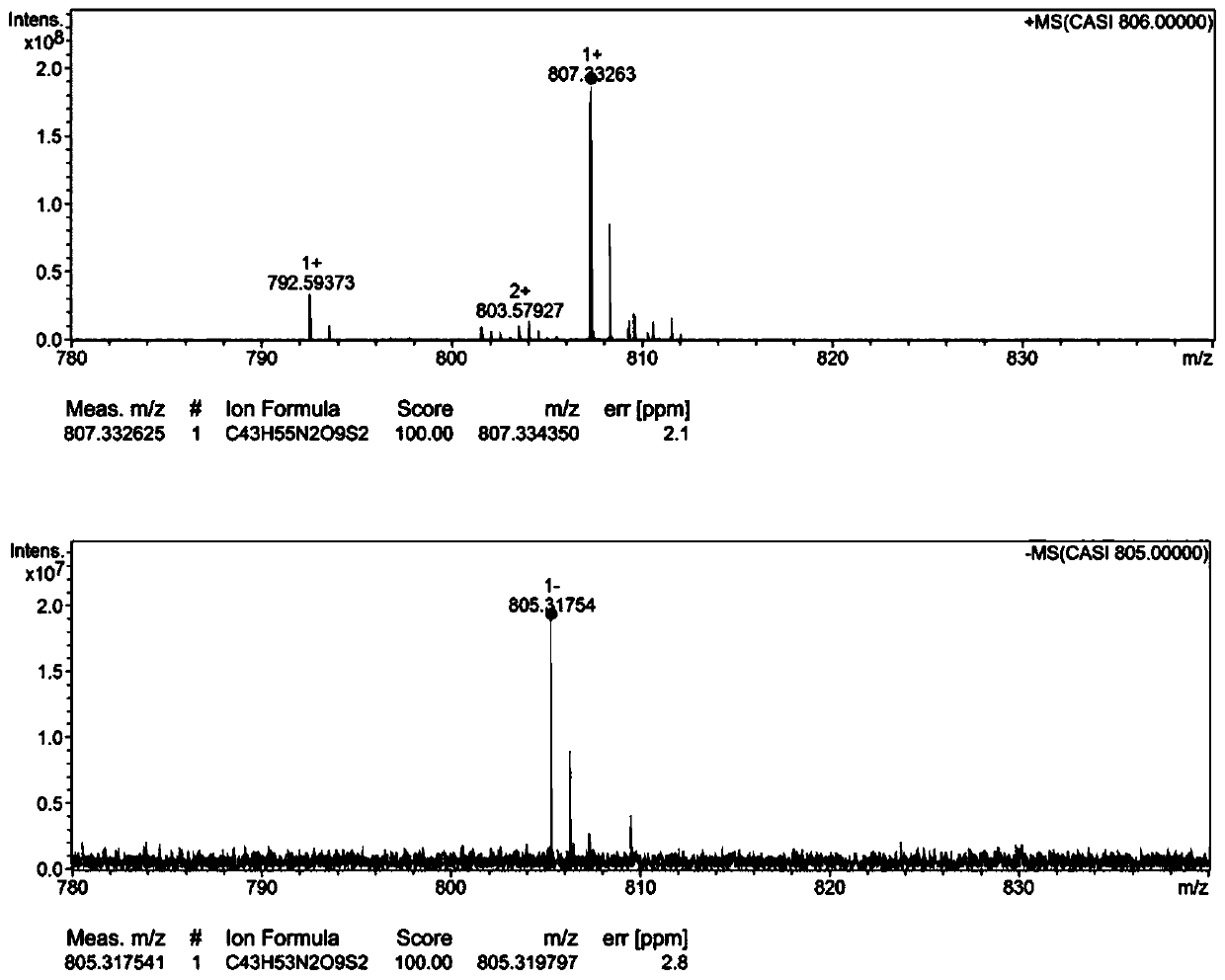
[0041] Embodiment 1: Synthesis of hydroxycamptothecin linoleate small molecule prodrug (HCPT-SS-LA)
[0042] Add linoleic acid to the toluene solution in which p-toluenesulfonic acid and 2,2'-dithiodiethanol have been dissolved, and the reaction system is in N 2 The temperature was raised to 110°C under protection. After the reaction was detected by TLC, the reaction was carried out with saturated NaHCO 3 solution extraction, followed by anhydrous Na 2 SO 4 The excess water was removed and the intermediate product was finally isolated by column chromatography. The intermediate product was dissolved in dichloromethane, DIPEA and p-nitrobenzoic chloroformate were added under ice-bath conditions, and stirred overnight at room temperature. Remove the solvent by rotary evaporation under reduced pressure, redissolve the system with DMF, cool it to 0°C in ice bath, slowly add the hydroxycamptothecin solution dissolved in DMF, add anhydrous triethylamine, react at room temperature...
Embodiment 2
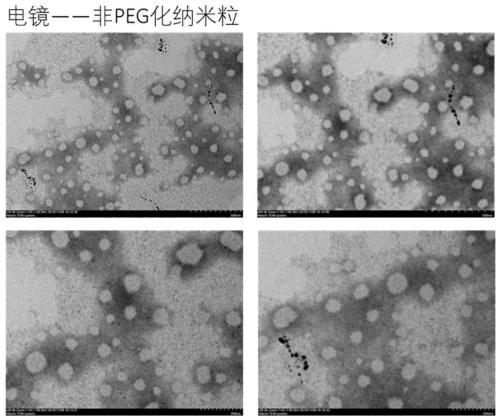
[0045] Example 2: Preparation of small molecule prodrug self-assembled nanoparticles
[0046] Precise weighing of TPGS 2k Appropriate amount (8% is 0.35 mg, 20% is 1 mg, 30% is 1.7 mg) and 4 mg of prodrug, dissolved in 1 mL of THF, slowly added the THF solution dropwise to 4 mL of deionized water under stirring, spontaneously formed Uniform nanoparticles (HCPT-SS-LA nanoparticles, HCPT-SS-LA / 8% PEG nanoparticles, HCPT-SS-LA / 20% PEG nanoparticles, HCPT-SS-LA / 30% PEG nanoparticles). Under the condition of 35°C, the organic solvent in the nano-preparation was removed by rotary evaporation under reduced pressure.
[0047] Table 1. Drug loading of hydroxycamptothecin linoleate prodrug
[0048]
[0049] The formulations in Table 1 can produce prodrug self-assembled nanoparticles, but the particle size of 30% PEGylated nanoparticles is not reproducible in different batches, fluctuating around 130-160nm, while 8% and 20% PEGylated There is almost no difference in particle size b...
Embodiment 3
[0054] Example 3: Cytotoxicity of PEG-modified small molecule prodrug self-assembled nanoparticles
[0055] The cytotoxicity of PEG-modified small molecule prodrug self-assembled nanoparticles on mouse breast cancer (4T1) cells was investigated by MTT assay. Digest the cells in good condition, dilute them with culture medium to a cell density of 1000cells / mL, blow evenly, add 200 μL of cell suspension to each well of a 96-well plate, and incubate in an incubator for 24 hours to make them adhere to the wall. After the cells adhere to the wall, add hydroxycamptothecin or the prodrug nanoparticles prepared in Example 2. In the experiment, the preparation and dilution of the drug solution and the nanoparticle preparation were all made with 1640 culture solution, and sterile filtered with a 0.22 μm filter membrane. 200 μL of the test solution was added to each well, and 3 parallel wells were prepared for each concentration. For the control group, without adding the drug solution ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com