Pharmaceutical composition for treating tumors or cancers and application thereof
A composition and tumor technology, applied in drug combination, anti-tumor drug, pharmaceutical formula, etc., can solve the problems of narrow anti-tumor spectrum of oncolytic virus, low antibody efficiency, and inconclusiveness, so as to improve tracking and killing ability , Elimination of immunosuppressive response, simple and stable genome effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Cancer: Cancers treated according to the combinations described herein include, but are not limited to, leukemia, acute lymphoblastic leukemia, acute myeloid leukemia, myeloblastic promyelocytic leukemia, myelomonocytic monocytic erythroleukemia, chronic leukemia , chronic myeloid (granulocytic) leukemia, chronic lymphocytic leukemia, mantle cell lymphoma, primary central nervous system lymphoma, Burkitt lymphoma and marginal zone B-cell lymphoma, polycythemia vera lymphoma, Chiggin's disease, non-Hodgkin's disease, multiple myeloma, Waldenstrom's macroglobulinemia, heavy chain disease, solid tumors, sarcomas, and carcinomas, fibrosarcomas , myxosarcoma, liposarcoma, chondrosarcoma, osteogenic sarcoma, osteosarcoma, chordoma, angiosarcoma, endothelial sarcoma, lymphangiosarcoma, lymphangioendothelioma, synovium, mesothelioma, Ewing's tumor, leiomyosarcoma , rhabdomyosarcoma, colon sarcoma, colorectal cancer, pancreatic cancer, breast cancer, ovarian cancer, prostate can...
Embodiment 2
[0094] Example 2 To establish a subcutaneous transplanted lung cancer model, and compare the therapeutic effects of different mutant strains of viruses in lung cancer:
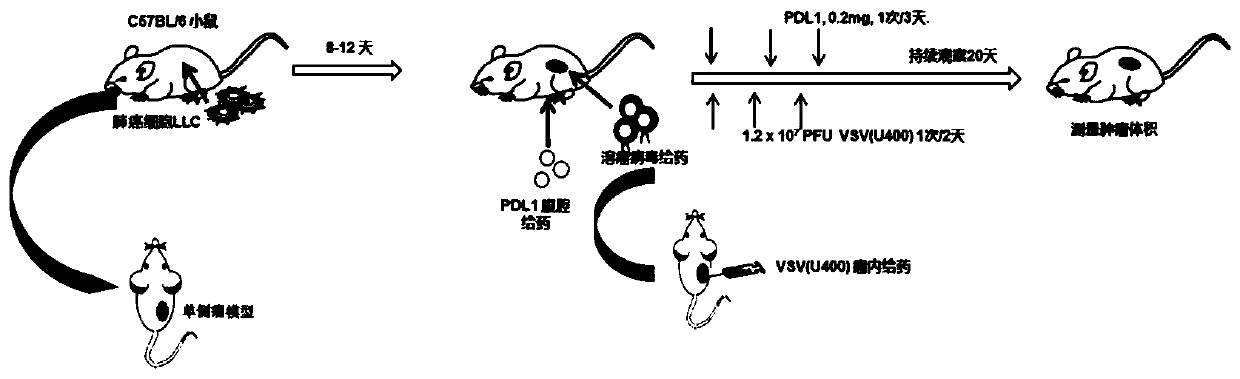
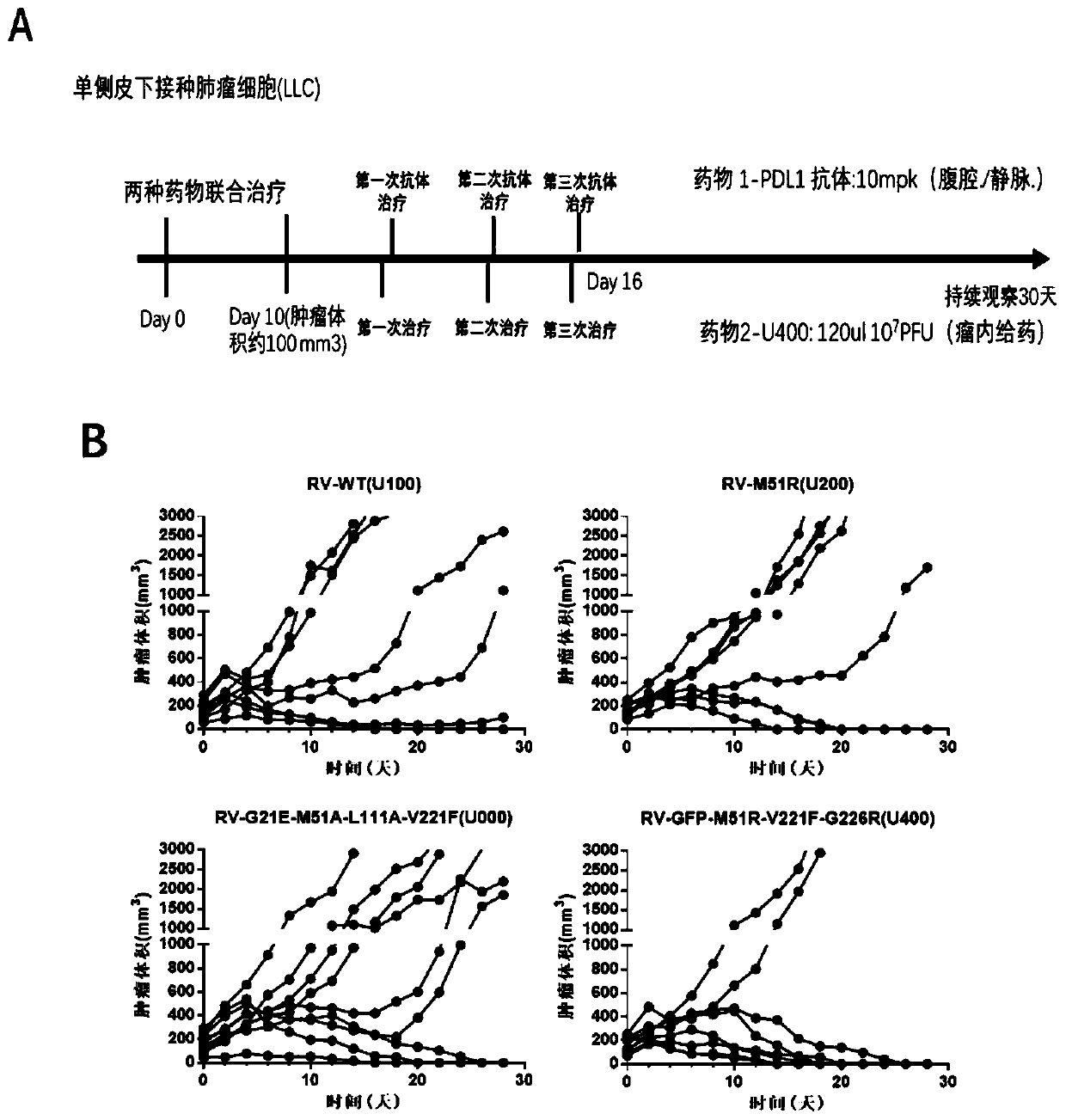
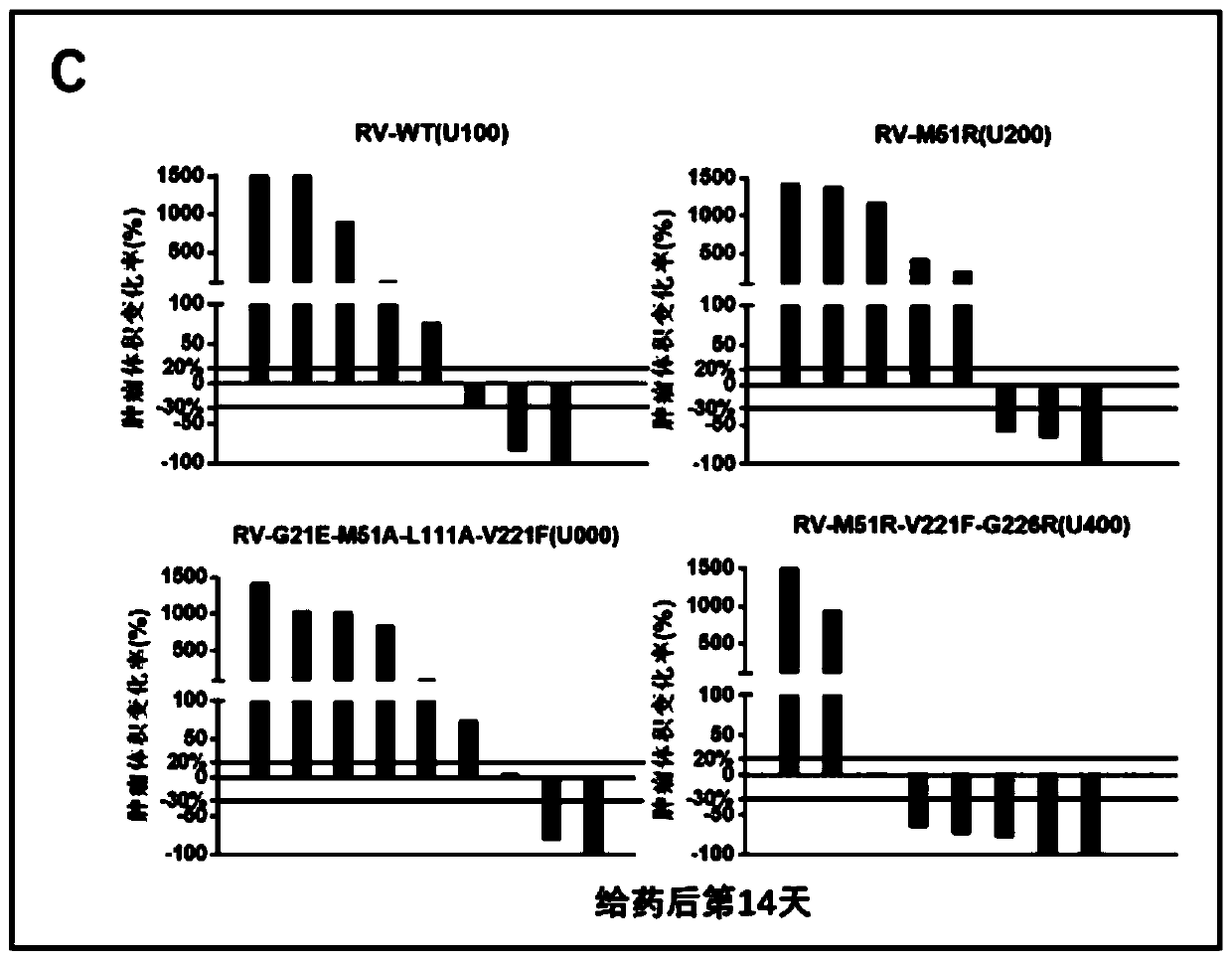
[0095] figure 2 A is the specific plan for intratumoral administration of oncolytic virus. As shown in the figure, after the tumor volume of lung cancer reaches at least 100mm3, intratumoral administration of oncolytic virus is given. The volume of intratumoral administration is 120ul, and the interval between administration is 1-2 days, depending on the original tumor growth rate. According to the above-mentioned administration flow chart, further in the mouse lung cancer tumor model, compare the therapeutic effect of different mutant strain viruses on lung cancer, figure 2 B shows the change in tumor volume of each tumor model mouse after administration of different mutant strains of virus. Further statistics show that compared with the wild-type virus U100, three mutant strains U200, U000 and U400 have i...
Embodiment 3
[0096] Example 3: Comparison of the killing ability of different viruses, especially U400, on tumor cells and the toxicity to normal cells: the in vitro killing of different mutant viruses on different cells was detected by the method of MTT detection.
[0097] The specific steps of the above detection method are as follows:
[0098] 1. Add (LLC / Hela / MC38 / MEF) cell suspension 100µl to each well of a 96-well culture plate to make the cell volume reach 1×10 4 1 / well, cultured at 37°C, 5% CO2 for 16 hours;
[0099]2. Dilute the viruses RV-WT (U100), RV-M51R (U200), RV-M51R-V221F-G226R (U400), RV-G21E-M51A-L111A-V221F (U000) to MOI (multiplicity of infection) respectively Inoculate 4 wells for each dilution gradient of 0.001, 0.01, 0.1, 1.0, 10, 100µl per well, and incubate at 37°C, 5% CO2 for 40h;
[0100] 3. Discard the supernatant in the 96-well culture plate, add fresh medium, and add MTT solution, 20 μL / well. Incubate at 37°C, 5% CO2 for 4 hours;
[0101] 4. Centrifuge th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com