Probe and method for detecting ESR2 gene polymorphism
A gene polymorphism and probe technology, which is applied in biochemical equipment and methods, microbial measurement/testing, DNA/RNA fragments, etc., can solve the problems of high cost, inapplicability of large sample detection, time-consuming and labor-intensive, etc., to achieve The effect of high sensitivity, clear genotyping, and simple method operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Construct and prepare the wild-type standard plasmid and mutant standard plasmid containing the target gene locus. The accuracy of the sequence can be confirmed by enzyme digestion and sequencing. The genotype of the wild-type standard plasmid rs1256054 is GG; the genotype of the mutant standard plasmid rs1256054 is CC. The standard plasmid DNA concentration was normalized to 10 ng / μL.
[0036] 2. Use the online software Primer 3 to design primers to strengthen the complementary sequence of the primer loop and the target DNA sequence. The sequence of the forward primer is: 5′-GCAACGGGTCACGTACGCAA TAGGGATGAGGGGAAATGCG-3′; the sequence of the reverse primer is: 5′-GCAACGGGTCACGTACGCAA CCCGATAAAACATGGCCCAG-3 '; the sequence of the probe is 5'-FAM-CGAGAGTTAAAACTCCAACACAAAGAATATCTCG-BHQ1-3'.
[0037] 3. Reaction system optimization:
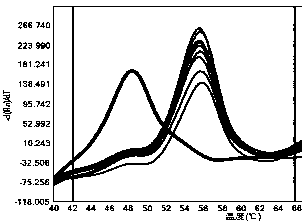
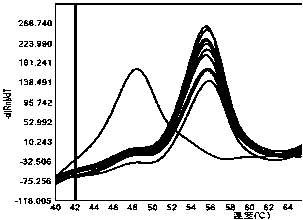
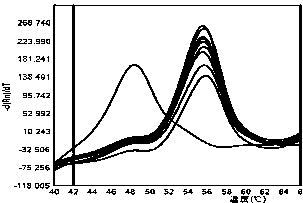
[0038] 1) Probe volume: set probe volumes of 0.01 μL, 0.05 μL, 0.1 μL and 0.5 μL respectively, and keep other conditions unchanged, and p...
Embodiment 2
[0045] 1. Using the silica gel adsorption method to extract the genomic DNA of the oral epithelial cells of a test subject, the concentration and purity of the DNA were detected by electrophoresis gel imaging, and the concentration of the sample to be tested was standardized to 10 ng / μL.
[0046] 2. The detection method is as follows: add 7.5 μL of PCR Mix, 0.3 μL of forward primer solution, 0.3 μL of reverse primer solution, and 0.1 μL of probe to the PCR reaction wells in sequence. Add 2 μL of DNA to each reaction well, make up 15 μL with sterilized double distilled water; carry out the reaction on the fluorescent quantitative PCR detection system SLAN-96P, the PCR reaction conditions are 95°C pre-denaturation for 5 minutes; 95°C denaturation for 30 seconds, 60°C annealing for 30 seconds, Extend at 72°C for 30 seconds, 45 cycles; extend at 72°C for 10 minutes; denature at 95°C for 1 minute, anneal at 40°C for 1 minute, monitor the fluorescence signal in real time during the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com