Method for constructing mouse preeclampsia model
A preeclampsia and model technology, applied in the field of biomedicine, can solve the problems of lack of research models, no pregnancy specificity, Gal-9 pathological changes and pathogenesis of preeclampsia, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
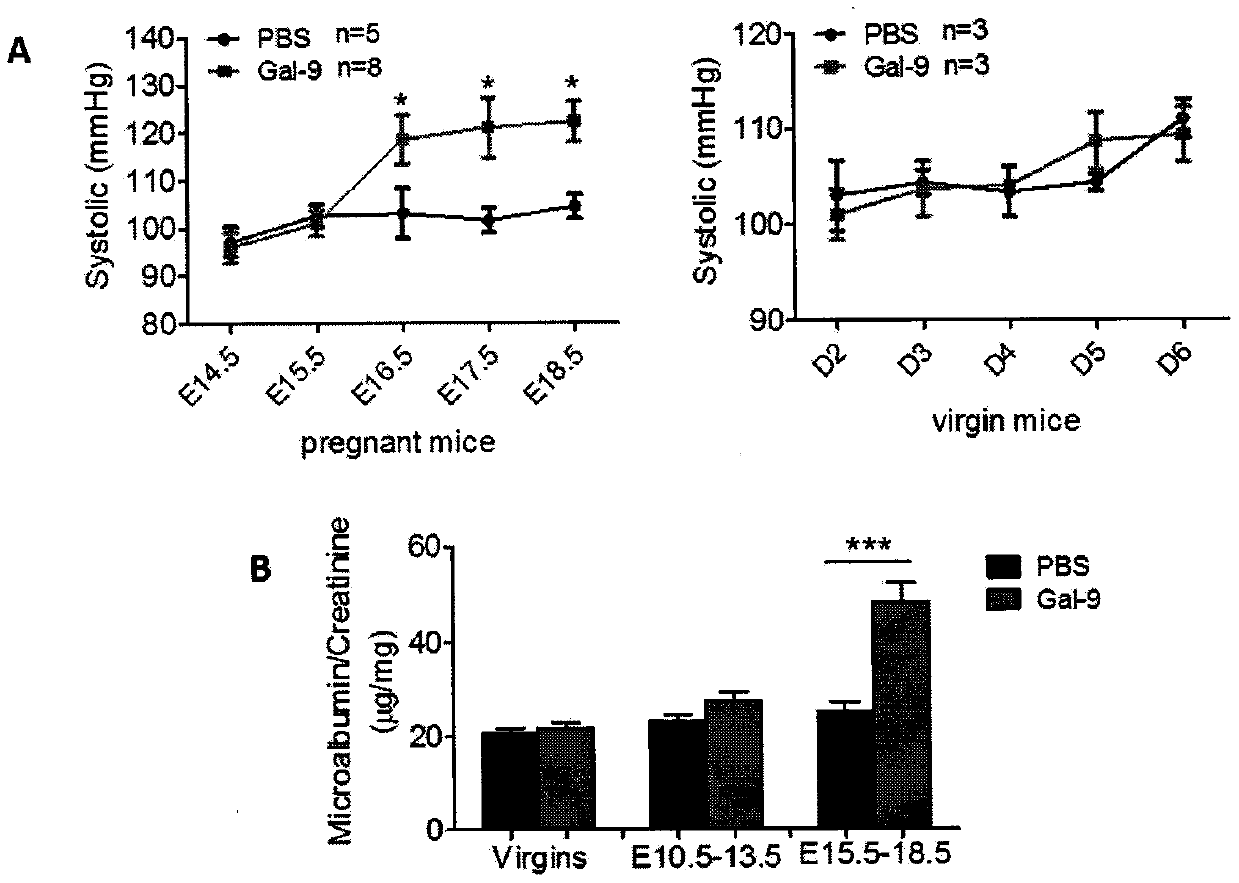
[0186] Example 1: Pre-eclampsia-related symptoms in pregnant mice induced by injection of recombinant Gal-9
[0187] Animals and diet: C57 / B6 mice of 6-8 weeks were purchased from Slack and raised in the experimental animal room of the Obstetrics and Gynecology Hospital of Fudan University. After adaptation, 80 C57 / B6 female mice with normal estrous cycle were housed with mature male mice of the same strain (2:1), and the vaginal plug was seen in the next morning as the mice were considered to be pregnant for 0.5 days;
[0188] Grouping and treatment of animals: 13 C57 / BL6 female mice on the 8.5th day of pregnancy were randomly divided into 5 mice in the control group (PBS) and 8 mice in the Gal-9 group; 10 non-pregnant C57 / BL6 female mice of the same age were also divided into There were 5 rats in each of the control group (PBS) and the Gal-9 group. Pregnant mice in the Gal-9 group were intraperitoneally injected with recombinant mice Gal-9 200 μg / mouse / day on the 8.5th, 10....
Embodiment 2
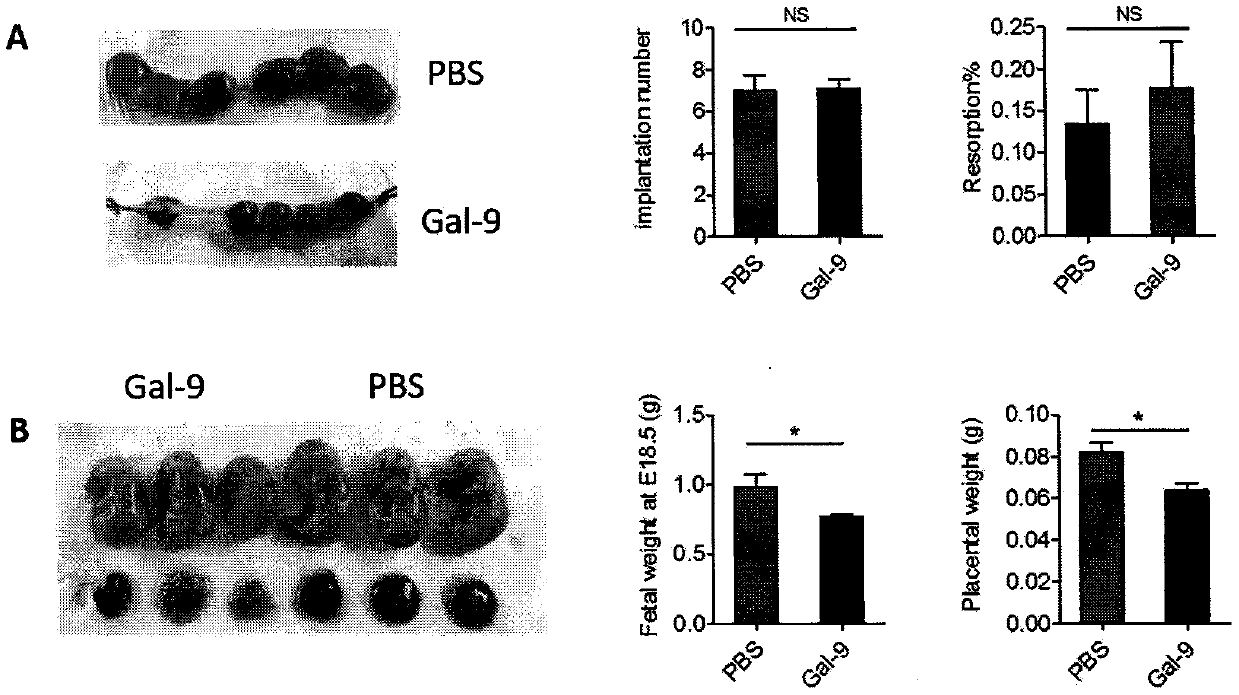
[0193] Example 2: Injection of recombinant Gal-9 causes the placenta and fetal mice of pregnant mice to shrink
[0194] Each group of female mice was treated as described in Example 1, and was killed at 18.5 days of pregnancy, the uterus was dissected, and the fetal mice and placenta were stripped; figure 2 As shown in A, the implantation number and embryo absorption rate of pregnant mice were not significantly different between the Gal-9 injection group and the control group. Such as figure 2 As shown in B, the size of the fetal mice and placenta in the Gal-9 injection group was significantly smaller than that of the control group, and the weight of the fetal mice and the wet weight of the placenta were significantly reduced, suggesting that the high expression of Gal-9 leads to restricted embryonic growth and development and poor placental development. combine figure 1 The results indicated that the exogenous administration of Gal-9 could lead to typical symptoms of pree...
Embodiment 3
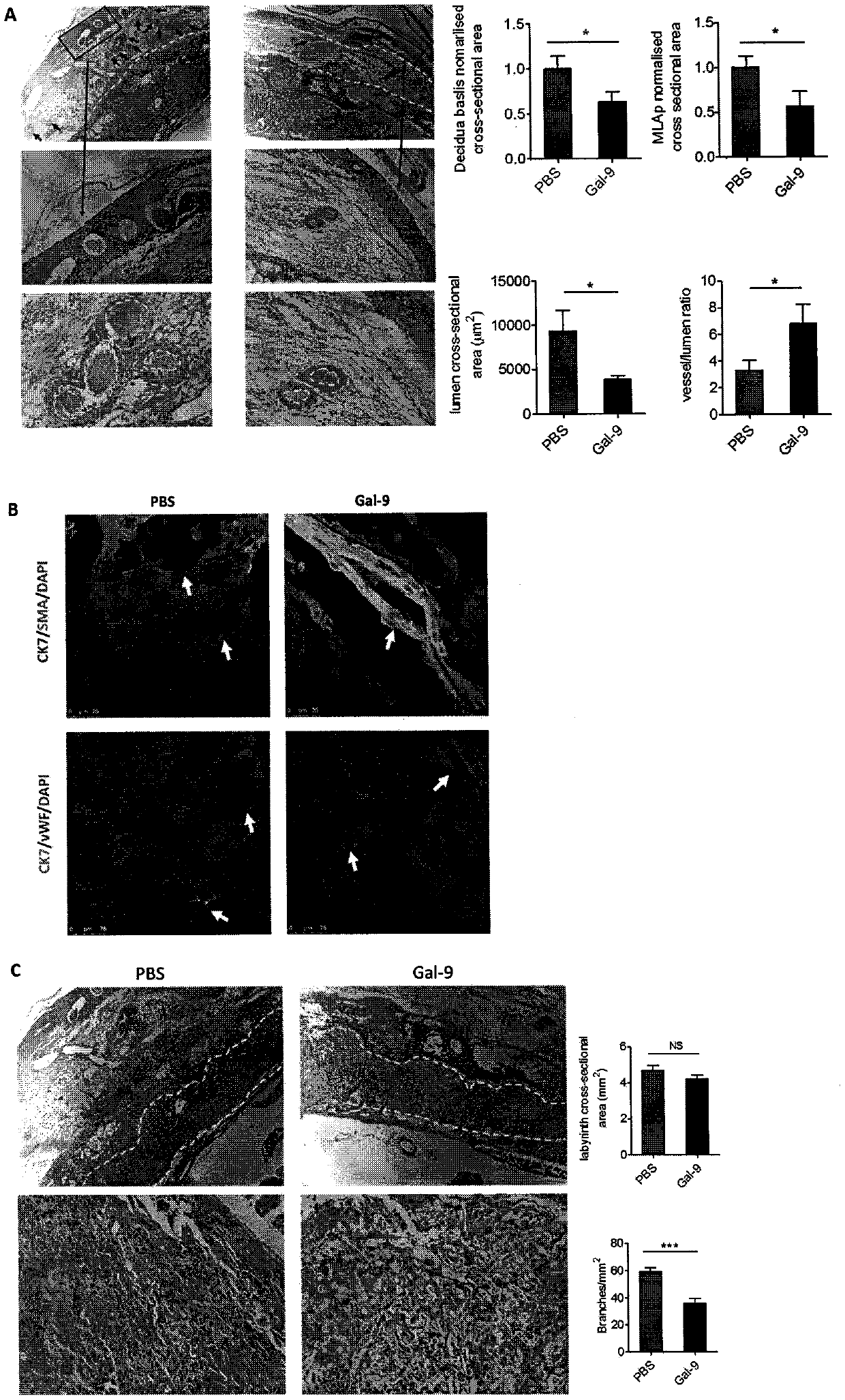
[0195] Example 3: Experiments of Gal-9 injection causing abnormal decidualization, poor spiral artery remodeling and destruction of placental labyrinth vascularization
[0196] Animals and diet: the same as in Example 1.
[0197] Grouping and treatment of animals: 16 C57 / BL6 female mice on the 8.5th day of pregnancy were randomly divided into 5 mice in the control group (PBS) and 8 mice in the Gal-9 group. Pregnant mice in the Gal-9 group were intraperitoneally injected with recombinant mice Gal-9 200 μg / mouse / day on days 8.5, 10.5, and 12.5 of pregnancy; pregnant mice in the control group were intraperitoneally injected with PBS 200 μl / mouse / day at the corresponding time. The mice were sacrificed on the 12.5th day of pregnancy, the uterus was dissected, the placenta was removed, and a complete pregnancy tissue of the implantation site (including the uterus, placenta, and fetal mouse) was fixed and embedded in paraffin or frozen OCT to prepare paraffin or the preparation of f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com