Preparation method of Fe2O3/C@Co2B catalyst and application of Fe2O3/C-coated Co2B catalyst in oxygen evolution reaction
A catalyst, fe2o3 technology, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as danger and long preparation process, so as to improve utilization rate, The process steps are simple and controllable, and the structure is stable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Preparation of Fe 2 o 3 / C carrier:
[0039] In a heat-resistant container, put 20 mmol of FeSO 4 Prepare 1000 mL of 20 mM FeSO by dissolving in deionized water 4 solution as electrolyte;
[0040] Spectrally pure graphite rods were used as the negative and positive electrodes, in which the diameter of the cathode was 28 mm, and the diameter of the anode was 8 mm; the distance between the two graphite rod electrodes was controlled to be 3 cm, and the two electrodes were arranged along the same horizontal line and immersed 3 cm below the solution.
[0041] A current of 60A was applied between the two electrodes, and the voltage was 23 V; the distance between the two electrodes was kept at 2 mm by manual adjustment to ensure the stability of the arc during the discharge process. The duration of each discharge is 3 min, and then the power supply is cut off and the electrolyte is cooled to room temperature, and the above discharge process is repeated 20 times. The c...
Embodiment 2
[0059] (1) Preparation of Fe 2 o 3 / C carrier:
[0060] In a heat-resistant container, put 20 mmol of FeSO 4 Prepare 1000 mL of 20 mM FeSO by dissolving in deionized water 4 solution as electrolyte;
[0061] Spectrally pure graphite rods were used as the negative and positive electrodes, in which the diameter of the cathode was 28 mm, and the diameter of the anode was 8 mm; the distance between the two graphite rod electrodes was controlled to be 3 cm, and the two electrodes were arranged along the same horizontal line and immersed 3 cm below the solution.
[0062] A current of 80A was applied between the two electrodes, and the voltage was 28V; the distance between the two electrodes was kept at 2 mm by manual adjustment to ensure the stability of the arc during the discharge process. The duration of each discharge is 3 min, and then the power supply is cut off and the electrolyte is cooled to room temperature, and the above discharge process is repeated 20 times. The ca...
Embodiment 3
[0064] Embodiment 3: the present embodiment adopts the Fe prepared in embodiment 1 2 o 3 / C carrier material;
[0065] 1 mmol of CoSO 4 ·7H 2 O was formulated into CoSO at a concentration of 0.01 M 4 ·7H 2 O solution, to CoSO 4 ·7H 2 Add 30 mg of Fe to the O solution 2 o 3 / C carrier material and mix well, record as A solution.
[0066] 3 mmol NaBH 4 Dissolve in 0.1 M NaOH solution to make 0.3 M NaBH 4 and 0.1 M NaOH mixed solution, recorded as B solution.
[0067] Then, add liquid B dropwise to liquid A which is being stirred on a magnetic stirrer, and continue stirring for 3 hours after the addition is completed.
[0068] Finally, the resulting precipitate was washed twice with deionized water and absolute ethanol respectively, put into a vacuum drying oven to dry for 6 h, and then the samples were collected to obtain Fe 2 o 3 / C@Co 2 B catalyst.
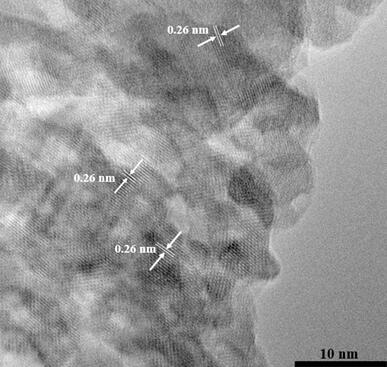
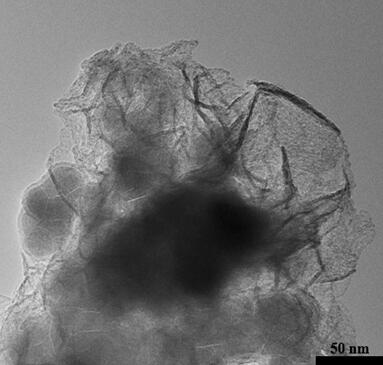
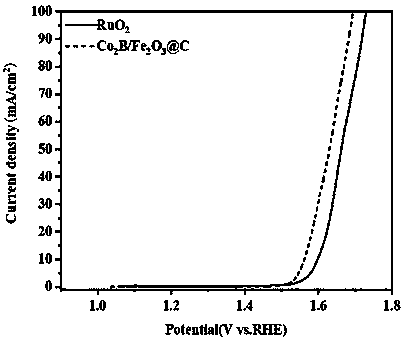
[0069] The Fe prepared by embodiment 3 2 o 3 / C@Co 2 The microscopic morphology of the B catalyst is a floccu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com