a cos x @mno 2 Composite material, preparation method and application thereof
A technology of composite materials and catalysts, applied in electrolytic components, electrodes, electrolytic processes, etc., can solve the problems of efficiency multi-step proton-coupled electron transfer obstacles, not widely used, overall efficiency obstacles, etc., to achieve excellent electrocatalytic performance and improve utilization rate, reducing the effect of aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A CoS X @MnO 2 The preparation method of catalyst, comprises the steps:
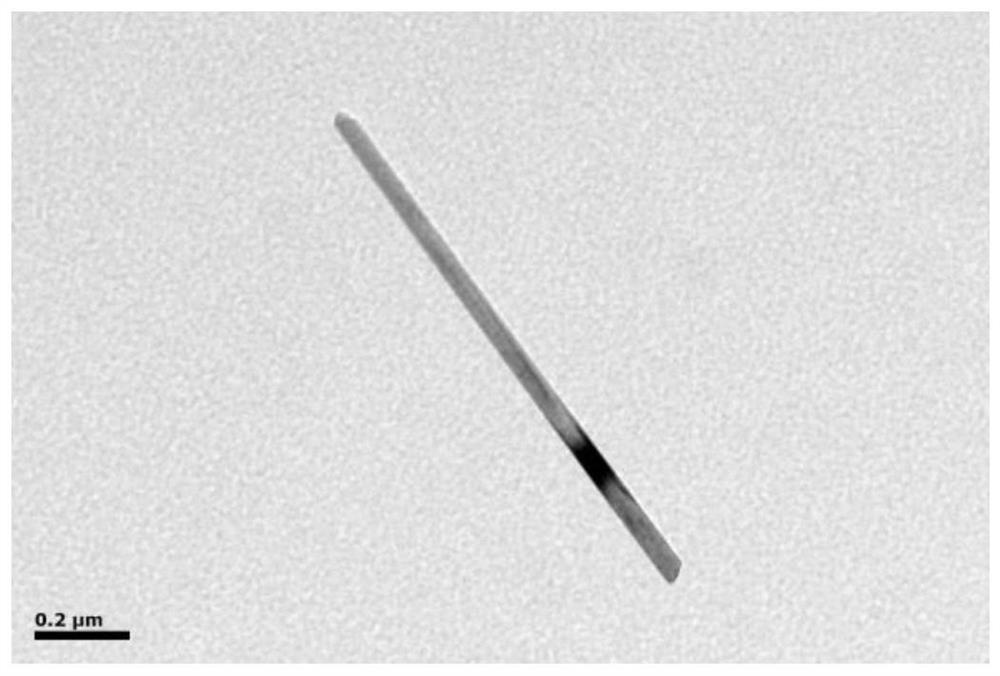
[0036] (1)MnO 2 Preparation of nanotubes: at room temperature, 0.3160g KMnO 4 Add to 30mL deionized water to disperse to form a uniform solution, and then make 10mL concentration 1moL·L -1 A solution of hydrochloric acid was added to the above KMnO 4 solution, stir and mix evenly, transfer the mixture to a hydrothermal reaction kettle, and react at 160°C for 5 hours, after the reaction is completed, centrifuge, wash with deionized water and absolute ethanol three times, and dry with electric blast at 60°C Oven drying for 8h to obtain the MnO 2 nanotube;
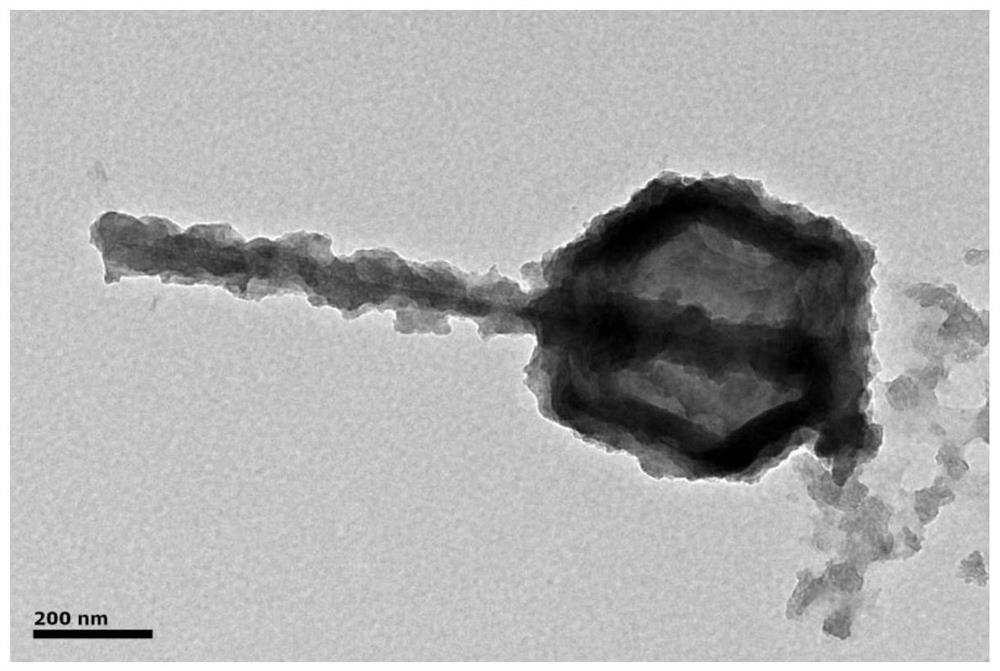
[0037] (2)ZIF-67@MnO 2 Preparation of composite material: first, the MnO prepared in step (1) 2 Add 0.0500 g of nanotubes into 25 mL of methanol for ultrasonic dispersion for 30 min, then stir magnetically for 30 min at room temperature, and then add 0.4000 g of Co(NO 3 ) 2 ·6H 2 O mix well, then quickly add 15 mL of CH containing 0.41...
Embodiment 2
[0040] A CoS X @MnO 2 The preparation method of catalyst, comprises the steps:
[0041] (1)MnO 2 Preparation of nanotubes: at room temperature, 0.5120g KMnO 4 Add to 50mL deionized water to disperse to form a uniform solution, and then make 12mL concentration to 1moL L -1 A solution of hydrochloric acid was added to the above KMnO 4 solution, stir and mix evenly, transfer the mixture to a hydrothermal reaction kettle, and react at 160°C for 5 hours, after the reaction is completed, centrifuge, wash with deionized water and absolute ethanol three times, and dry with electric blast at 60°C Oven drying for 8h to obtain the MnO 2 nanotube;
[0042] (2)ZIF-67@MnO 2 Preparation of composite material: first, the MnO prepared in step (1) 2 Add 0.0800 g of nanotubes into 30 mL of methanol for ultrasonic dispersion for 45 min, then stir magnetically for 30 min at room temperature, and then add 0.6000 g of Co(NO 3 ) 2 ·6H 2 O mix well, then quickly add 20 mL of CH containing 0...
Embodiment 3
[0045] A CoS X @MnO 2 The preparation method of catalyst, comprises the steps:
[0046] (1)MnO 2 Preparation of nanotubes: in parts by weight, at room temperature, 0.8310g KMnO 4 Add to 60mL deionized water to disperse to form a homogeneous solution, and then make 15mL concentration to 1moL L -1 A solution of hydrochloric acid was added to the above KMnO 4 solution, stir and mix evenly, transfer the mixture to a hydrothermal reaction kettle, and react at 160°C for 5 hours, after the reaction is completed, centrifuge, wash with deionized water and absolute ethanol three times, and dry with electric blast at 60°C Oven drying for 8h to obtain the MnO 2 nanotube;
[0047] (2)ZIF-67@MnO 2 Preparation of composite material: first, the MnO prepared in step (1) 2 Add 0.0900 g of nanotubes into 35 mL of methanol for ultrasonic dispersion for 30 min, then stir magnetically for 30 min at room temperature, and then add 0.8000 g of Co(NO 3 ) 2 ·6H 2 O mix well, then quickly add ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com