Method for long-term stable preparation of 4-chlorobutyryl chloride
A chlorobutyryl chloride, stable technology, applied in the field of long-term stable preparation of 4-chlorobutyryl chloride, can solve the problems of low yield and difficulty in industrial scale-up production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
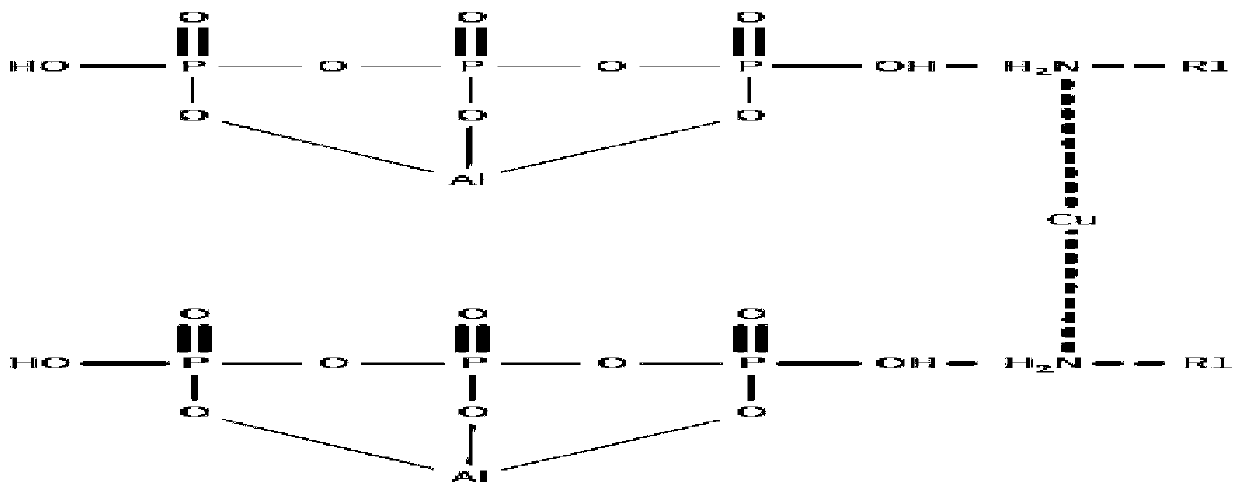
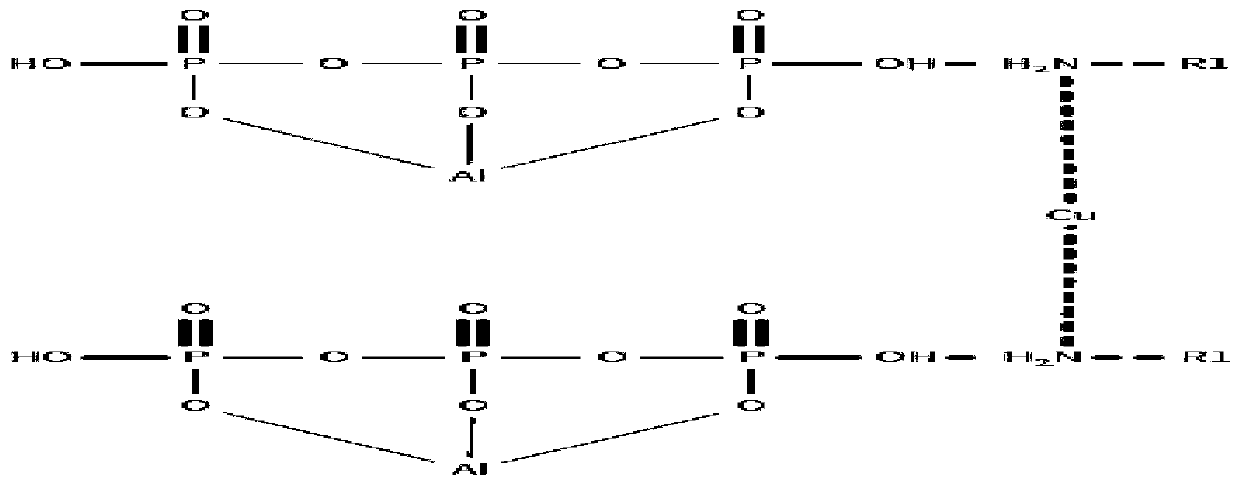
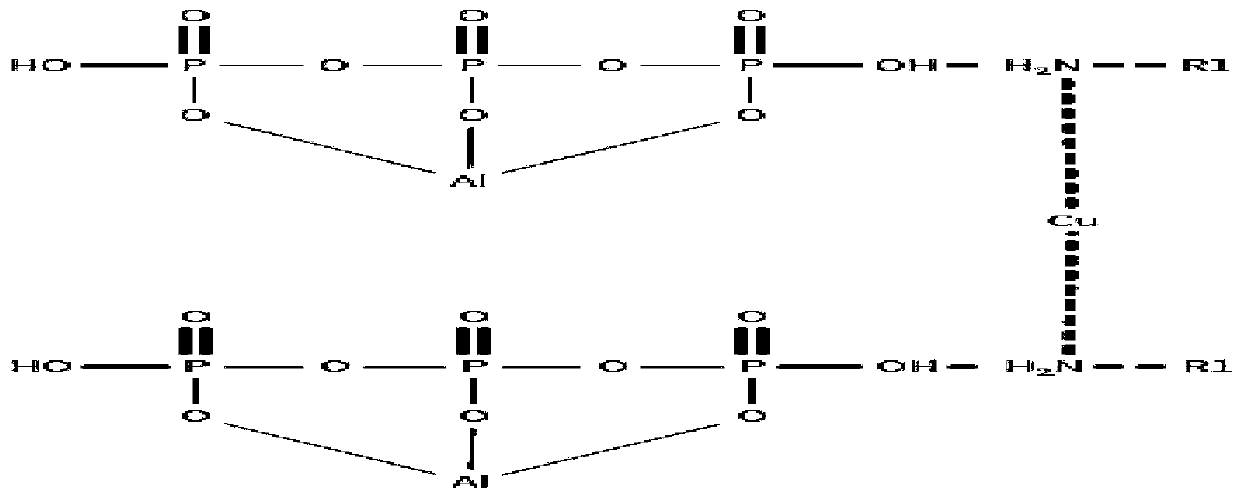
[0035] Add 9g of methylamine and 50ml of absolute ethanol into the flask, stir evenly, then drop into it an ethanol solution containing 16g of anhydrous copper sulfate, stir and react at room temperature, after the reactant disappears, add 100g of The ethanol solution of aluminum dihydrogen phosphate was stirred and reacted at room temperature, separated and treated to obtain 79.8g of catalyst A.
preparation example 2
[0037] According to the same method as catalyst preparation example 1, except that 14 g of ethylamine was added instead of methylamine, and ethyl acetate was used as the solvent, the rest were the same to obtain 82.7 catalyst B.
preparation example 3
[0039] According to the same method as catalyst preparation example 1, except adding 28g of aniline instead of methylamine, adding 14g of anhydrous copper chloride instead of copper sulfate, the rest were the same to obtain 96.6g of catalyst C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com