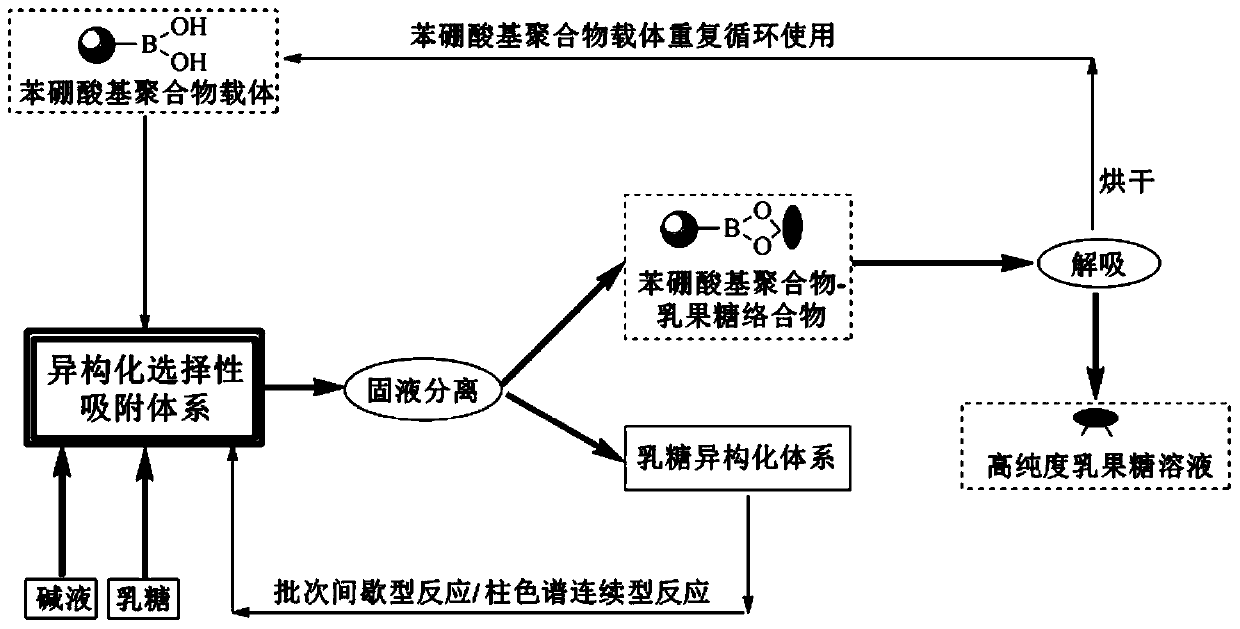
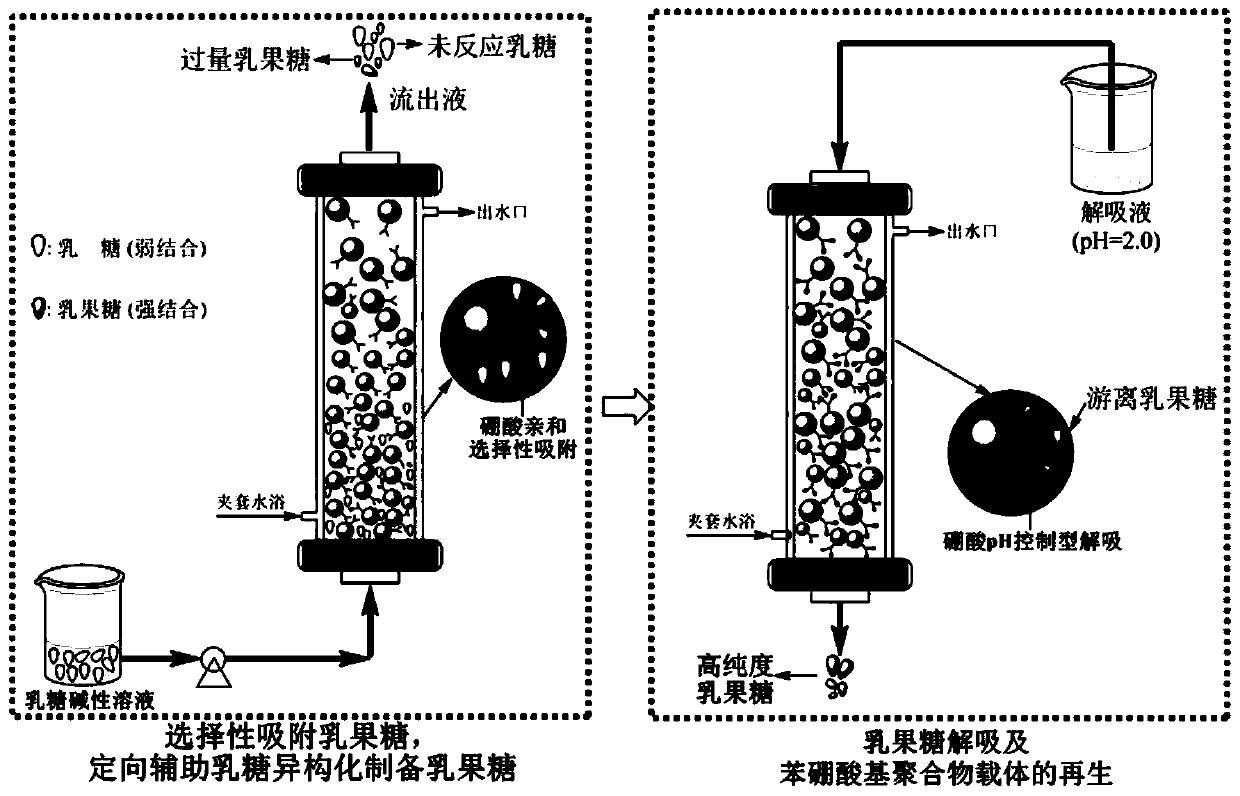
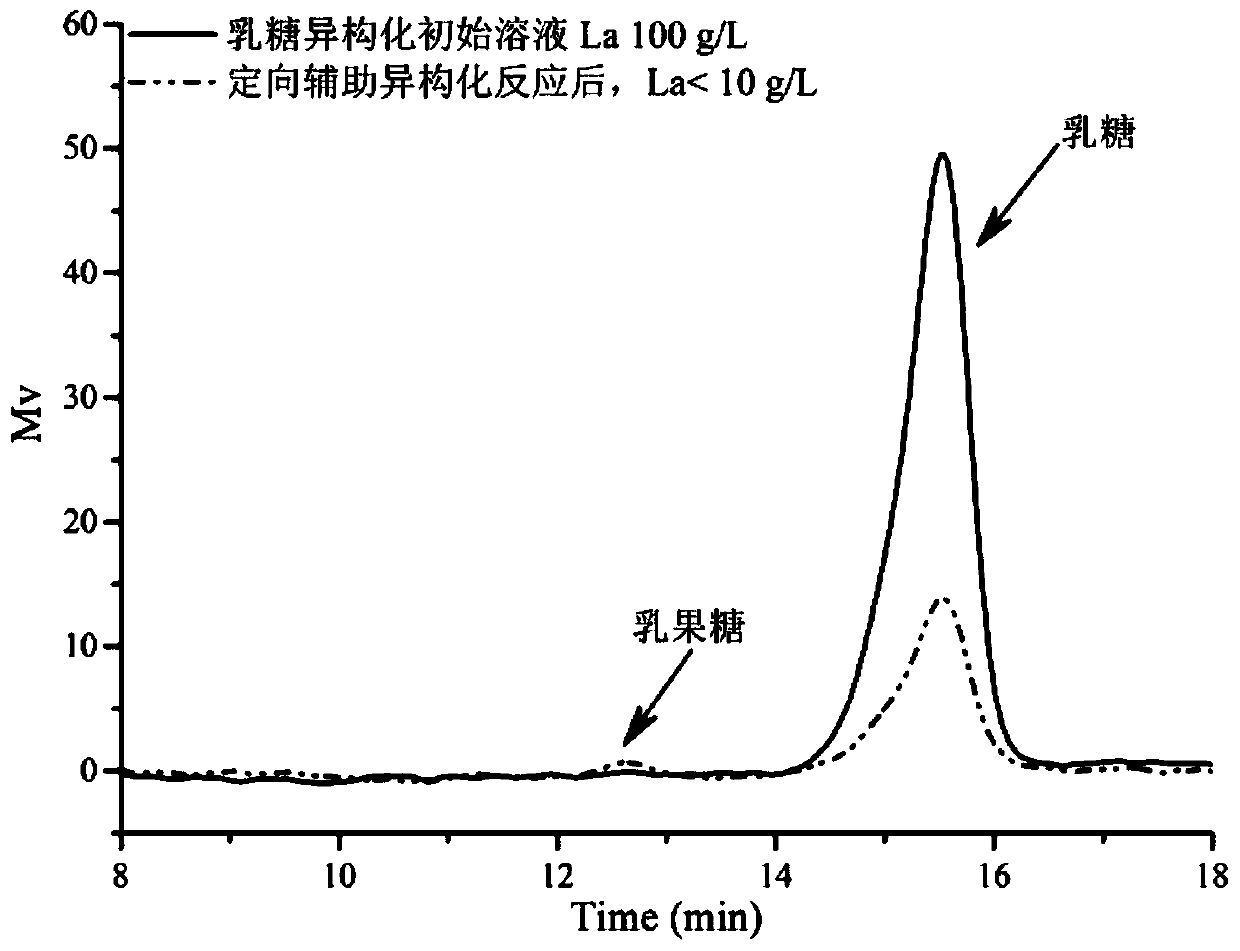
Method for preparing, separating and purifying lactulose by directionally assisting lactose isomerization by using phenylboronic acid-based carrier
A technology for separation and purification of phenylboronic acid, applied in the preparation of sugar derivatives, chemical instruments and methods, disaccharides, etc., can solve the problems of low recovery rate, many by-products, long time, etc., to improve yield and improve conversion rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Preparation of Lactulose by Isomerization of Lactose Assisted in Batch Mode
[0056] Dissolve 100g of lactose into 900mL of disodium hydrogen phosphate buffer solution (concentration: 0.5M), add NaOH to adjust the pH of the system to 11.0, and then add deionized water until the volume of the whole system is 1L. This solution is used as a lactose isomerization system solution.
[0057] Add 10g of phenylboronic acid-based carrier at the initial stage of the reaction, react at 60°C and 150rpm for 50min, remove the adsorbed phenylboronic acid-based carrier by suction filtration, and then add new 10g of phenylboronic acid-based carrier to the lactose isomerization system solution, Continue the above operations until the lactose content in the final system is lower than 10g / L.
[0058] Compared with the lactulose conversion rate of only about 15% under the single NaOH catalytic system, the final lactulose conversion rate can reach about 55% by using the above-ment...
Embodiment 2
[0059] Example 2: Preparation of Lactulose by Isomerization of Lactose Assisted in Batch Mode
[0060] Dissolve 100g of lactose into 900mL of disodium hydrogen phosphate buffer solution (concentration: 0.5M), add NaOH to adjust the pH of the system to 11.0, and then add deionized water until the volume of the whole system is 1L. This solution is used as a lactose isomerization system solution.
[0061] Add 10g of phenylboronic acid-based polymer carrier at the initial stage of the reaction, react for 25min at 60°C and 150rpm, remove the adsorbed phenylboronic acid-based polymer carrier by suction filtration, and then add new 10g of phenylboronic acid-based polymer carrier to the lactose isomerization system solution. Boric acid-based polymer carrier and 10-15g lactose (25min lactose conversion rate is about 15%-20%), continue the above operation, stop sugar supplementation after 10 rounds of sugar supplementation cycles, and use the mode in Example 1 to operate until the final...
Embodiment 3
[0063] Example 3: Preparation of Lactulose by Column Chromatography Continuous Mode Assisted Isomerization of Lactose
[0064] Dissolve 250g of lactose into 800mL of disodium hydrogen phosphate buffer solution (concentration: 0.5M), add NaOH to adjust the pH of the system to 11.0, and then add deionized water until the volume of the whole system is 1L. This solution is lactose isomerization System solution, as the next feed liquid.
[0065] Load 60-80g of phenylboronic acid-based carriers into four 100cm-long chromatographic columns, raise the temperature of the feed liquid to 50°C, and then slowly pass it into No. 1 chromatographic column from the bottom of the chromatographic column, making it flow through Carrier, after 30 minutes of the uppermost layer of liquid, stop feeding the feed liquid to the No. 1 chromatographic column, and then pass it into the No. 2 chromatographic column, and do the same until all 4 chromatographic columns are fully adsorbed and saturated. At th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com