Amphiphilic Lindqvist-type polyacid TiO2 composite nanofiber as well as preparation method and application thereof
A composite nanofiber and nanofiber technology, applied in chemical instruments and methods, refining with oxygen-containing compounds, organic compound/hydride/coordination complex catalyst, etc., can solve problems such as weak repeatability and multi-acid agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
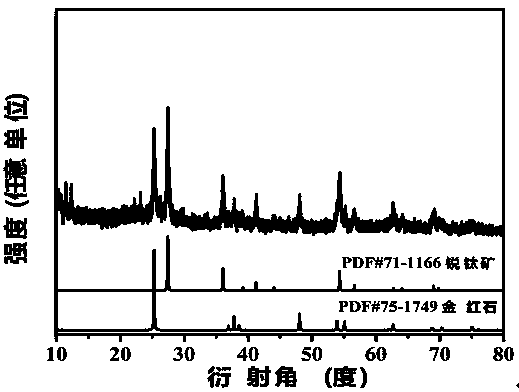
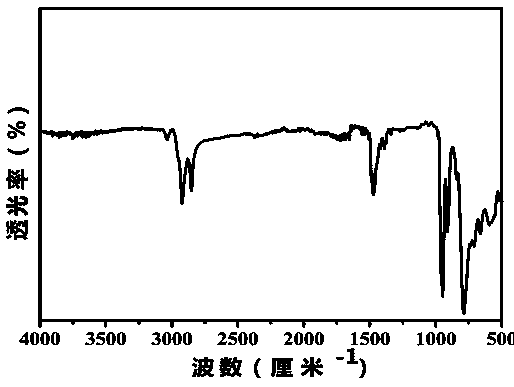
Image
Examples
Embodiment 1
[0036] Embodiment 1 (C 15 h 34 N) 2 (Mo 6 o 19 ) / TiO 2 Preparation of composite nanofibers
[0037] 1. (C 15 h 34 N) 2 (Mo 6 o 19 )Synthesis:
[0038] 2.50 g Na 2 MoO 6 2H 2 O (10.3 mmol) dissolved in 10~20 mL water, with 6mol L -1 HCl solution (2.5~3.0mL) was acidified, stirred vigorously for 1~5 min, added 2 mL of aqueous solution containing 1.16g (3.75 mmol) dodecyltrimethylammonium bromide, and heated the mixed solution to 75~80 °C, Heating for 40~50min, filtering the resulting precipitate and washing with ether 2~3 times, drying for 8~12h, to obtain (C 15 h 34 N) 2 (Mo 6 o 19 );
[0039] 2. TiO2 2 Synthesis of Nanofibers:
[0040] Using butyl titanate as a titanium source, it was completely dissolved in a mixed solvent formed by N,N-dimethylformamide, glacial acetic acid and acetylacetone, and then polyvinylpyrrolidone with a molecular weight of 1,300,000 was added as a template agent, using Electrospinning technology to get TiO 2 Nanofibers;
[0...
Embodiment 2
[0045] Example 2 Extraction Catalytic Oxidative Desulfurization Experiment of Simulated Oil Containing Dibenzothiophene
[0046] Completely dissolve 0.10g of biphenyl and 0.21g of dibenzothiophene in 100mL of n-octane to obtain 100mL of simulated oil containing 500ppm of dibenzothiophene; first, add 5 mL of simulated oil sample into a 25mL round bottom flask , keep the temperature in a water bath at 40-80 ℃ for 15 min; then, add 10.21-30.63 μL H 2 o 2 , 0.005-0.02g (C 15 h 34 N) 2 (Mo 6 o 19 ) / TiO 2 Composite nanofibers and 1 mL of ionic liquid [Bmim]PF 6 , magnetically stirred for 10-90min, and the upper oil sample was taken out every 10min for gas chromatography analysis, the results showed that: adding 11μL of H 2 o 2 , 0.01g at 60°C (C 15 h 34 N) 2 (Mo 6 o 19 ) / TiO 2 100% desulfurization efficiency can be achieved within 40 minutes, see the experimental results Figure 4 shown; after the reaction, the upper layer of simulated oil was dumped as much as possi...
Embodiment 3
[0047] Embodiment 3 (C 15 h 34 N) 2 (Mo 6 o 19 ) / TiO 2 Preparation of composite nanofibers
[0048] 1. (C 19 h 42 N) 2 (Mo 6 o 19 )Synthesis:
[0049] 2.50 g Na 2 MoO 6 2H 2 Dissolve O (10.3 mmol) in 10-20 mL of water, acidify with 2.5-3.0 mL of HCl solution, stir vigorously for 1-5 min, add 2 mL of cetyltrimethylammonium bromide containing 1.37 g (3.75 mmol) The aqueous solution, the mixed solution was heated to 75 ~ 80 ℃, heated for 40 ~ 50min, the resulting precipitate was filtered and washed with ether 2 ~ 3 times, dried for 8 ~ 12h, that is (C 19 h 42 N) 2 (Mo 6 o 19 );
[0050] 2. TiO2 2 Synthesis of Nanofibers:
[0051] Using butyl titanate as a titanium source, it was completely dissolved in a mixed solvent formed by N,N-dimethylformamide, glacial acetic acid and acetylacetone, and then polyvinylpyrrolidone with a molecular weight of 1,300,000 was added as a template agent, using Electrospinning technology to get TiO 2 Nanofibers;
[0052] 3. (C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com