Aminobenzodifuran diketone-based oligomer, preparation method and application thereof, and organic field effect transistor
A furandione-based and furandione technology is applied in the field of organic field effect transistors, which can solve the problems of incapable of taking into account the intermolecular self-assembly characteristics, intermolecular ordered aggregation characteristics and carrier migration performance, etc., and achieves excellent thermal stability. and solubility, strong donor-acceptor exchange performance, excellent molecular planarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
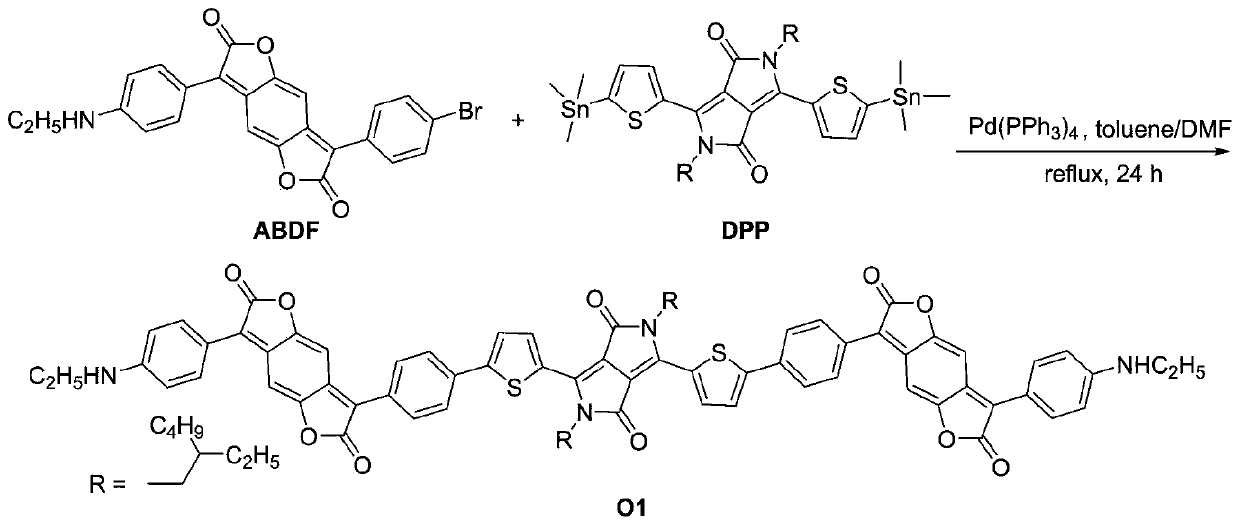
[0037] The present invention also provides a method for preparing the aminobenzodifurandione-based oligomer described in the above technical scheme, comprising the following steps:
[0038] dissolving aminobenzodifurandione and bisthiophene-substituted diketopyrrolopyrrole in a mixed solvent, and performing the first anhydrous and oxygen-free operation on the obtained mixed system to obtain a mixed solution;
[0039] The mixed solution is mixed with tetrakistriphenylphosphine palladium, followed by the second anhydrous and anaerobic operation, Stille coupling reaction and purification treatment to obtain the aminobenzodifurandione-based oligomer.
[0040] In the present invention, unless otherwise specified, each component in the preparation method is a commercially available product well known to those skilled in the art.
[0041] In the present invention, aminobenzodifurandione and bisthiophene-substituted diketopyrrolopyrrole are dissolved in a mixed solvent, and the obtain...
Embodiment 1
[0080] Aminobenzodifurandione: According to the literature "Z.Deng, K.Yang, L.Li, W.Bao, X.Hao, T.Ai, K.Kou. Solution processed air-stable p-channel organic crystal field -effecttransistors ofAminobenzodifuranone.Dyes and Pigments, 2018,151,173-178"prepared,
[0081] Bithiophene-substituted diketopyrrolopyrrole: purchased from Sarn Chemical Technology (Shanghai) Co., Ltd., tetrakistriphenylphosphine palladium: purchased from Sarn Chemical Technology (Shanghai) Co., Ltd.;
[0082] Dissolve 152.6mg (0.33mmol) of aminobenzodifurandione and 127.5mg (0.15mmol) of bisthiophene-substituted diketopyrrolopyrrole in 15mL of a mixed solvent, wherein the mixed solvent is toluene and N,N-dimethyl Formamide is mixed at a volume ratio of 4:1, and the resulting mixed system is mixed under N with a flow rate of 10 sccm 2 And carry out the first water-free and oxygen-free operation for 10 minutes under the condition of ultrasound to obtain the mixed solution;
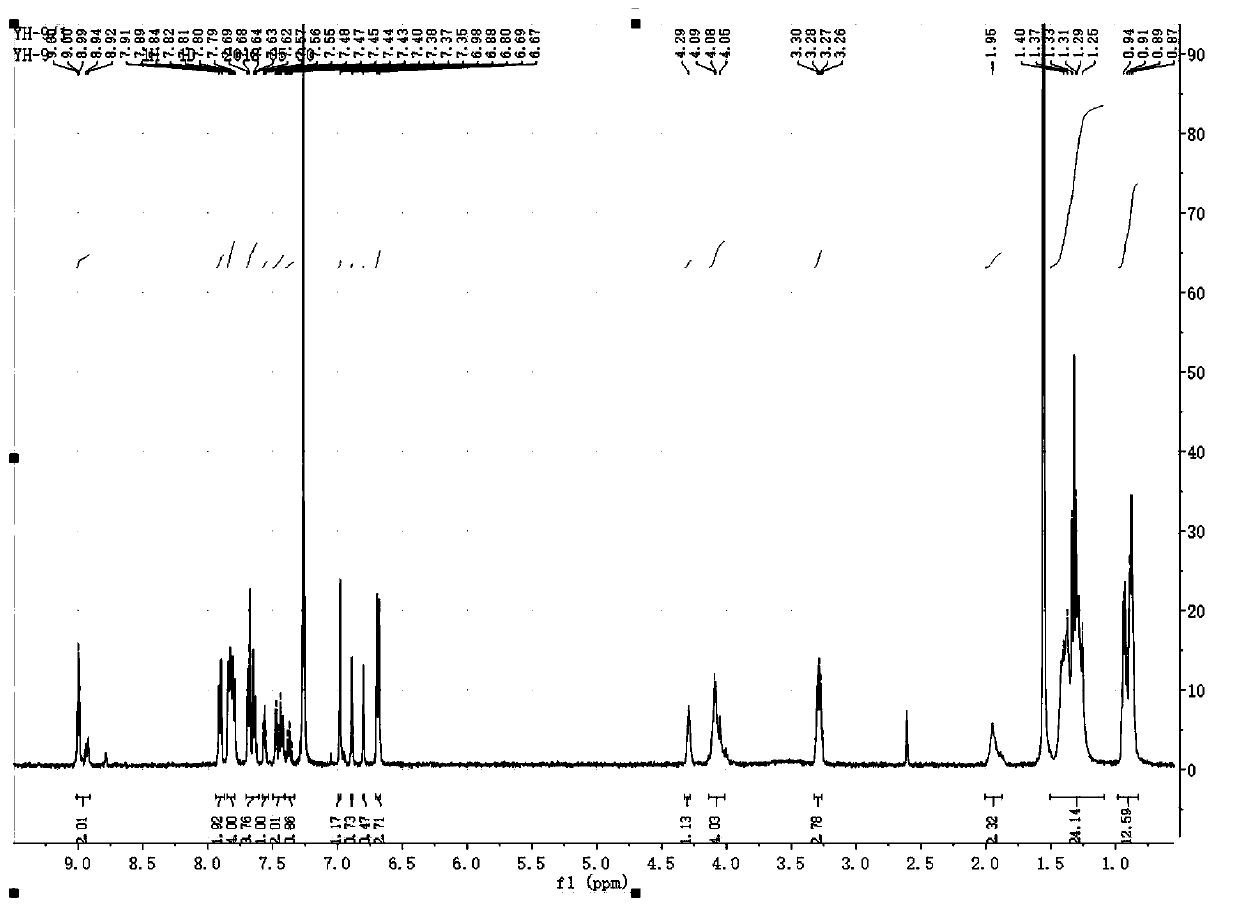
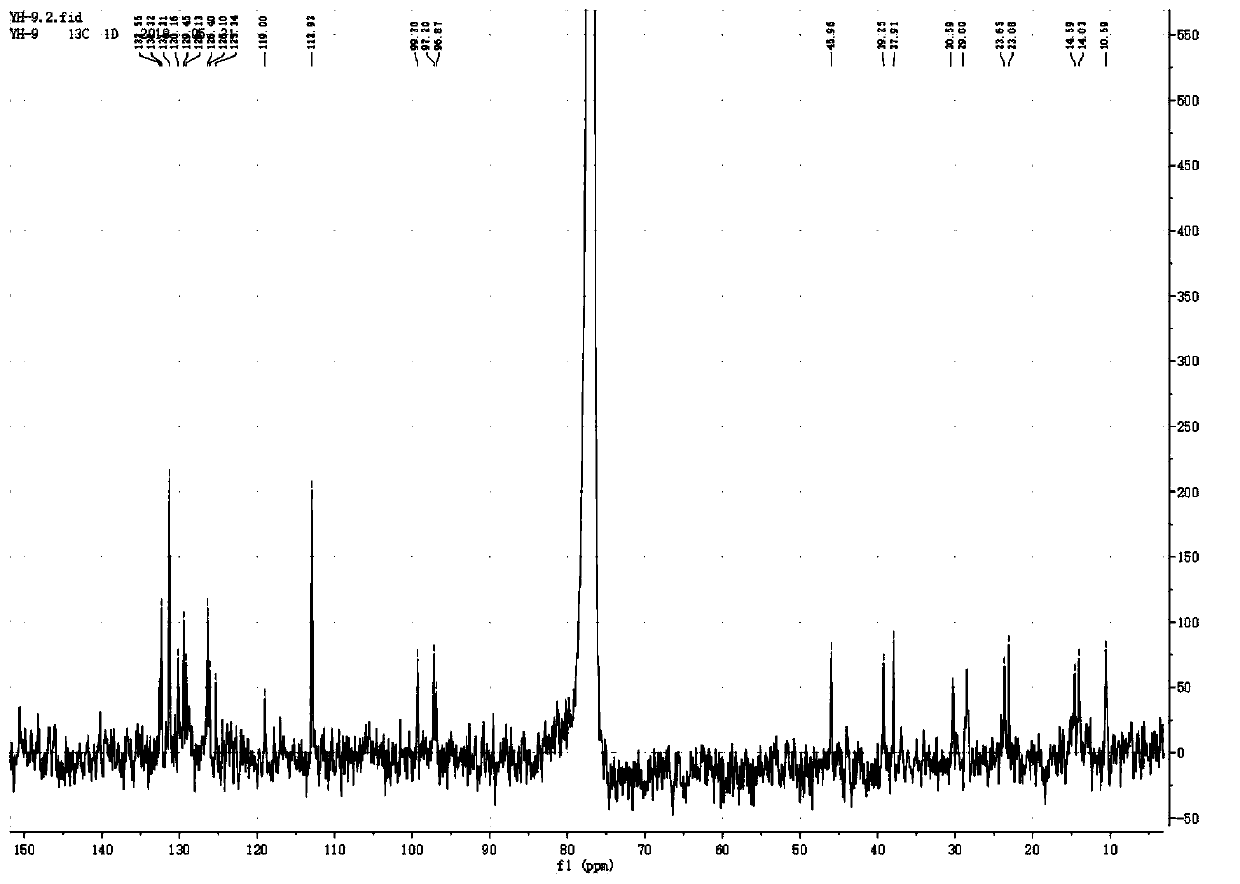
[0083] in N 2 Add 8mg (0.0075m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com