Device for preparing proteomics micro-sample
A sample preparation and trace technology, applied in the field of proteomics, to achieve the effect of superior performance, important application value and simple production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1. Fabrication and activation of proteomics micro-sample preparation device
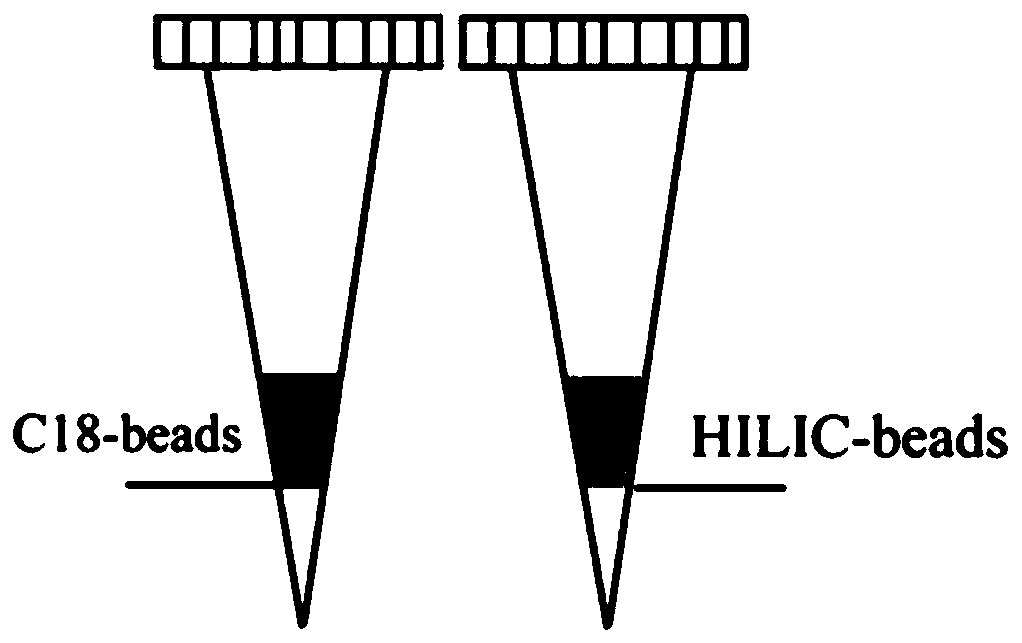
[0050] (1) Production of C18-Tip: Add a layer of C8 membrane as a sieve to the 200μl pipette tip, introduce 1mg of C18 filler above the sieve, and finally add a layer of sieve above the C18 filler.
[0051] (2) The above C18-Tip activation: First, add 100μl of pure acetonitrile (ACN, Merck) to C18-Tip, centrifuge at 1500g for 30s to remove the liquid; secondly, add 100μl of 50% ACN+0.1% TFA (trifluoro Acetic acid, Sigma) solution was centrifuged at 1500g for 60s to remove the liquid; finally, 100μl of 0.1% TFA solution was added to the C18-Tip and centrifuged at 1500g for 90s to remove the liquid.
[0052] (3) Production of HILIC-Tip: Add a layer of C8 membrane as a sieve plate to a 200μl pipette tip, and introduce 0.5 mg of HILIC filler above the sieve plate to make an enrichment column.
[0053] (4) The above HILIC-Tip activation: first, add 100μl 1% TFA solution to HILIC-Tip, centrifuge at ...
Embodiment 2
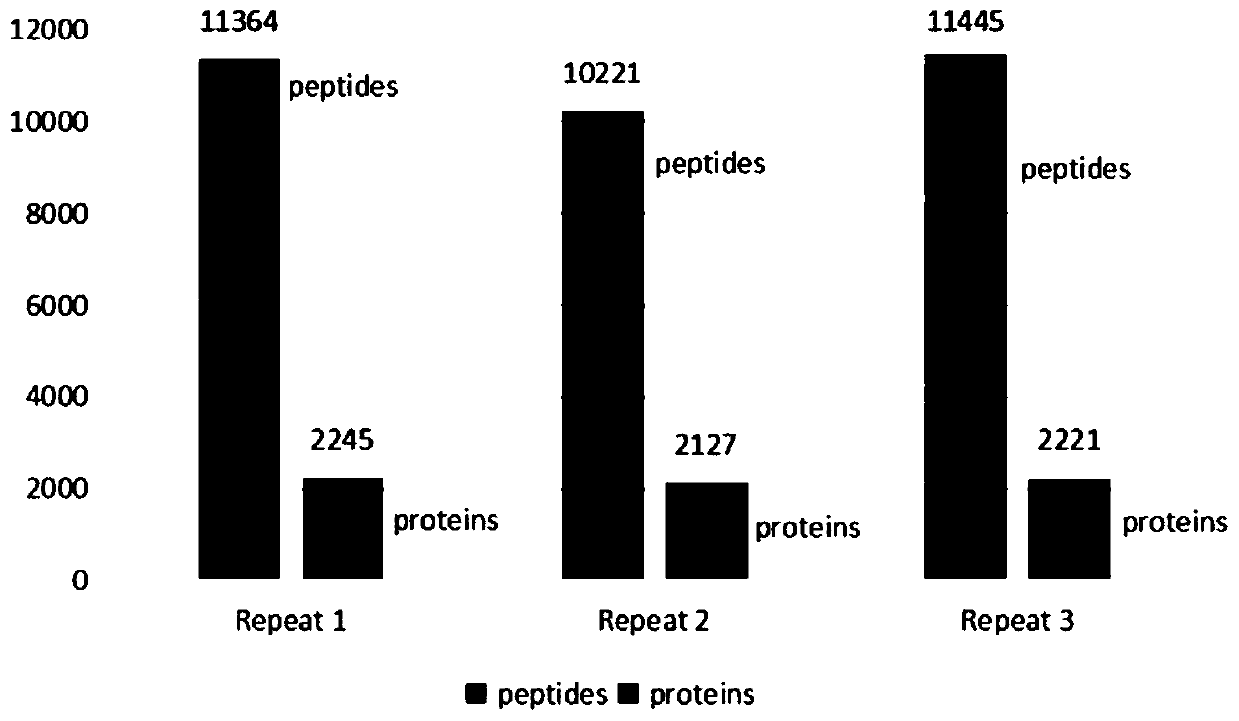
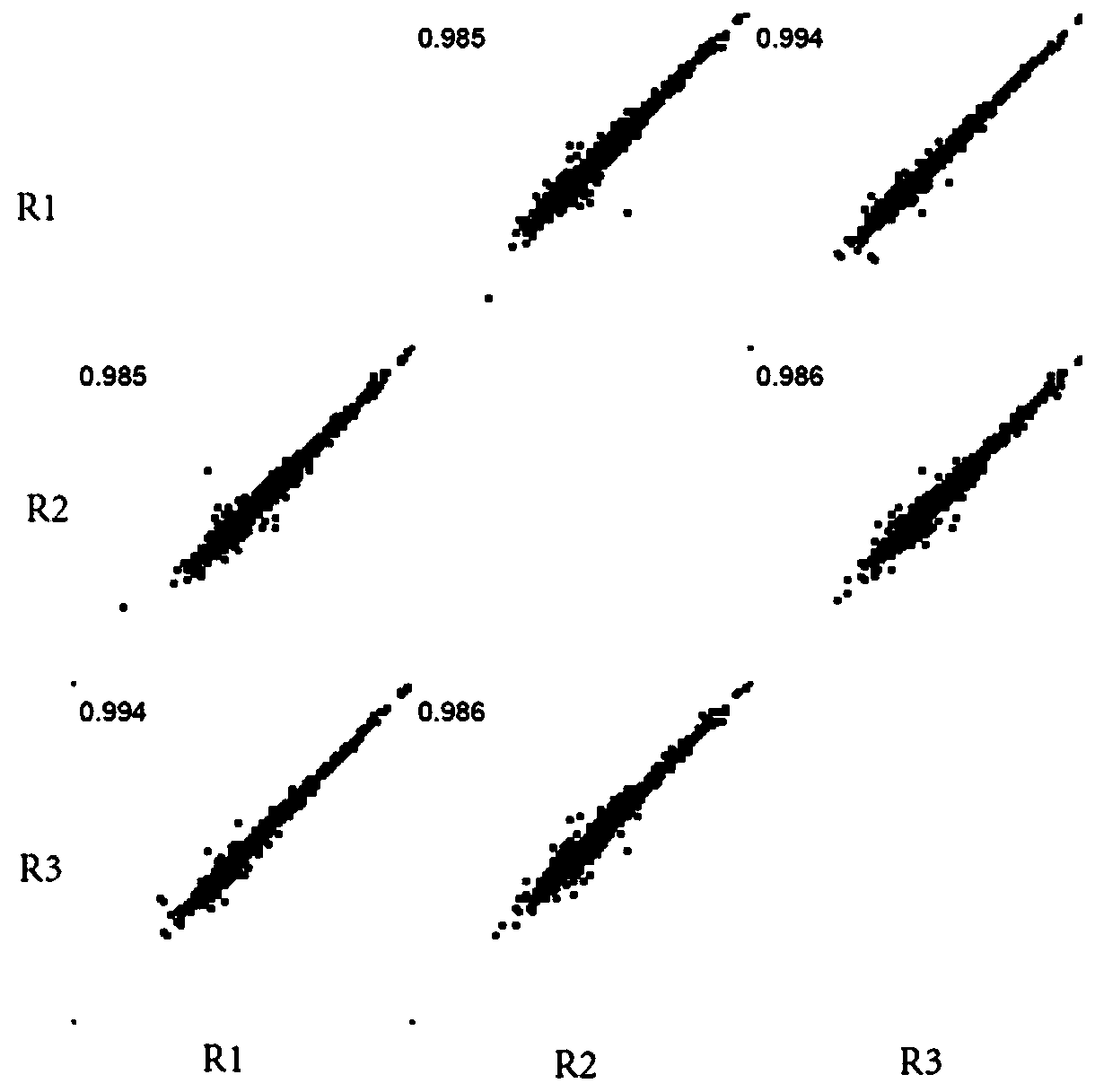
[0056] Example 2. Whole protein digestion experiment of human liver cancer HepG2 cell line
[0057] The whole protein of human liver cancer HepG2 cell line (purchased from American Model Culture Collection) was extracted with 8M urea (UA, Sigma) solution, and the protein concentration was measured by Bradford method.
[0058] Take 10μg of protein and place it in C18-Tip, add tris(2-carboxyethyl)phosphine (TCEP, Sigma), the final concentration is 10mM, add chloroacetamide (CAA, Sigma), the final concentration is 15mM, pipetting and mixing, The reduction reaction and the alkylation reaction were carried out simultaneously for 1 h at 37°C. Both TCEP and CAA were dissolved with 8M UA.
[0059] Add intracellular protease (Lys-C, Spec. Act:14.6Unit / (min*mg), 250mM*2775μl Ac-Lys-pNA and 20μg / in the enzyme / protein ratio of 1:100(w / w) into the solution. mL*225μl protease, incubate at 30°C for 30min, and measure absorbance at 405nm (mOD / min) over time. The maximum slope of the curve is used...
Embodiment 3
[0069] Example 3. Human immunoglobulin G (IgG) sugar cutting and enrichment experiments
[0070] 1) Human immunoglobulin G (IgG) dissolved in 50mM NH 4 HCO 3 in.
[0071] 2) Take 10μg of IgG in C18-Tip, add tris(2-carboxyethyl)phosphine (TCEP), the final concentration is 10mM, add chloroacetamide (CAA), the final concentration is 15mM, pipetting and mixing, at 37℃ At the same time, the reduction reaction and the alkylation reaction were carried out for 1 h.
[0072] 3) Trypsin was added to the solution at an enzyme / protein ratio of 1:100 (w / w), and incubated at 37°C for 16 hours to digest IgG.
[0073] 4) Add peptide-N-glycosidase F (PNGase F, Activity:>) to the solution at an enzyme / protein ratio of 1:20 (w / w) 1000u / mg, the unit of enzyme activity per milligram of protein. ), incubate at 37°C for 2h to release N-sugar chains.
[0074] 5) Add TFA to make the TFA concentration in the digestion solution 0.1%, centrifuge at 1500g for 3 minutes, and collect N-sugar chains.
[0075] 6) Add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com