Phosphorothioate modified nucleic acid aptamer medicine conjugate as well as a preparation method and application thereof
A technology of full phosphorothioate and nucleic acid aptamer, which can be applied in the field of medicine and can solve problems such as reduced efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
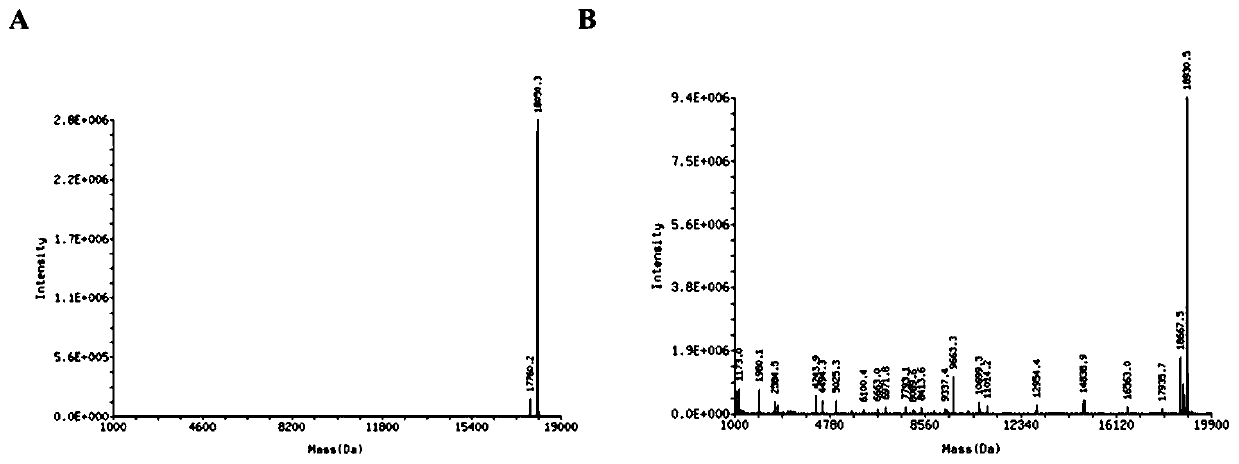
[0044] The second aspect of the present invention provides a method for preparing the all-phosphorothioate-modified nucleic acid aptamer-drug conjugate provided in the first aspect of the present invention, comprising: linking the drug molecule with a nucleic acid fragment substituted by a phosphorus-sulfur bond to provide The method for providing the phosphorothioate-modified nucleic acid aptamer-drug conjugate with phosphorus-sulfur bond-substituted nucleic acid fragments can specifically refer to the literature methods given above.
[0045] In the preparation method provided by the present invention, the preparation method may specifically include: 1) modifying the drug molecule with a linker molecule; 2) reacting the drug molecule modified with the linker molecule with a nucleic acid fragment substituted with a phosphorus-sulfur bond to provide the All phosphorothioate-modified nucleic acid aptamer-drug conjugates.
[0046] In the preparation method provided by the present...
Embodiment 1
[0056] A. Preparation of 4-nitrophenyl-4-(2-dimercaptopyridyl) ethyl carbonate:
[0057]
[0058] Add 88mg (0.4mmol) 2,2'-pyridine dimercapto, 31.3mg (0.4mmol) 2-mercaptoethanol, and 10mL dichloromethane into a 50mL three-neck round bottom flask. Stirring at room temperature for 2h, the reaction process was monitored by TLC. Dichloromethane was removed by rotary evaporation under reduced pressure, and purified by silica gel column reaction to obtain 20 mg of 2-(2-dimercaptopyridyl)ethanol as a yellow-white oily liquid with a yield of 53%.
[0059] Add 18.7mg (0.1mmol) 2-(2 dimercaptopyridyl) ethanol to 50mL three-neck round bottom flask, add 10mL anhydrous dichloromethane, add p-nitrophenyl chloroformate 40mg (0.2mmol), N , N'-diisopropylethylamine 25.9mg (0.2mmol), 4-dimethylaminopyridine 1.22mg (catalytic amount), stirred overnight at room temperature, and the reaction process was monitored by TLC. After the reaction is complete, add 0.01M hydrochloric acid solution to ...
Embodiment 2
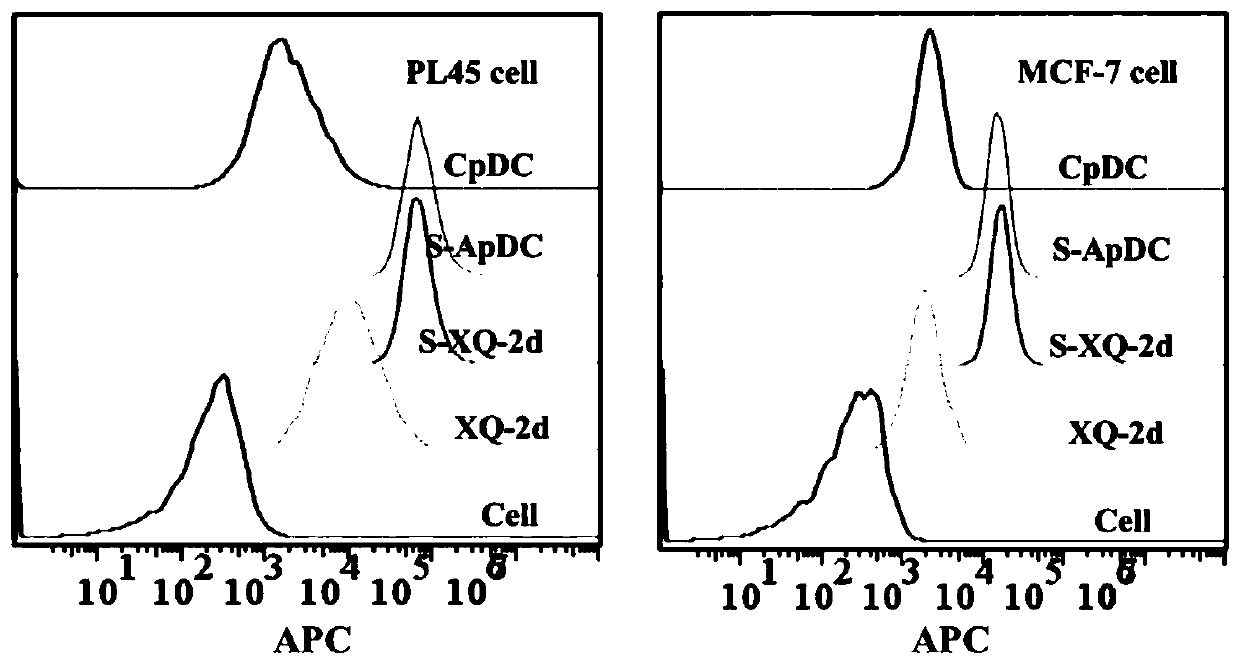
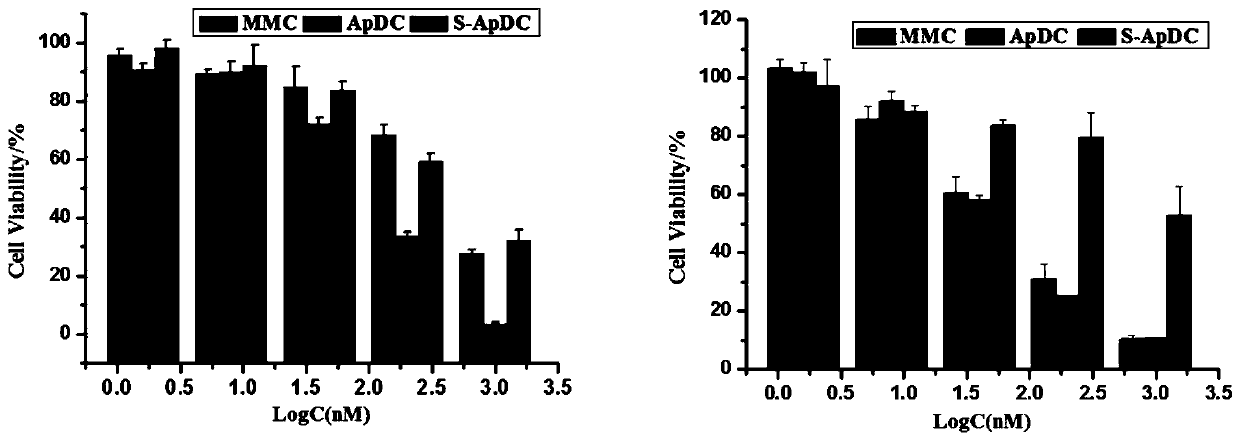
[0067] The targeted uptake ability of the thionucleic acid aptamer-mitomycin C conjugate by tumor cells:
[0068] Further investigate the ability of the thionucleic acid aptamer drug conjugate S-ApDC (prepared in Example 1) to selectively enter tumor cells, and at the same time add the control sequence drug conjugate CpDC (5'-ATTGCACTTACTATATTGCACTTACTATATTGCACTTACTAT-3', SEQ ID NO .2, not thioxated, the preparation method refers to Example 1, only the nucleic acid sequence is different). PL45 cells or MCF-7 cells were digested and inoculated into 12-well plates at a density of 100,000 / dish. After culturing in a 37°C incubator for 24 hours, they were washed three times with DPBS, and then 200 μL of 250 nM Cy5-labeled single-chain (XQ- 2d or S-XQ-2d, unthio aptamer and thio aptamer respectively, neither drug-conjugated, SEQ ID NO.1) and S-ApDC / CpDC, incubated at 37°C for 2h and washed with DPBS After 3 times, it was digested with trypsin for 1-2min, and detected by flow cytome...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com