Application of Haloferula sp. beta-N-acetylhexosaminidase to synthesis of breast milk oligosaccharide
A technology of amino acid and lactose, which is applied in the application field of synthesizing human milk oligosaccharides, can solve the problems that there is no report on the production of β-N-acetylhexosaminidase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1, excavation of β-N-acetylhexosaminidase gene and construction of engineering bacteria
[0078] 1. Determination of β-N-acetylhexosaminidase gene
[0079] The protein sequence of the reported β-N-acetylhexosaminidase was searched through the CAZy database, and the sequences were compared and analyzed by homologous sequences, and the β-N-acetylamino group of a glycoside hydrolase 20 family derived from Haloferula sp. Hexosidase gene (designated HaHex74). The nucleotide sequence of the HaHex74 gene is "ATG+No. 280-2238 of SEQ ID No.3", encoding the amino acid sequence shown in "Met+No. 94-745 of SEQ ID No.2". HaHex74 gene had the highest homology of 61% with a soil metagenome β-N-acetylhexosaminidase (AKC34129). Then send it to Shanghai Sangon Biological Co., Ltd. to synthesize the gene.
[0080] 2. Construction of engineering bacteria expressing β-N-acetylhexosaminidase
[0081] 2.1. Design primers according to the sequence of the glyceraldehyde triphosph...
Embodiment 2
[0090] Embodiment 2, preparation of β-N-acetylhexosaminidase and its enzymatic properties
[0091] 1. High copy screening of β-N-acetylhexosaminidase
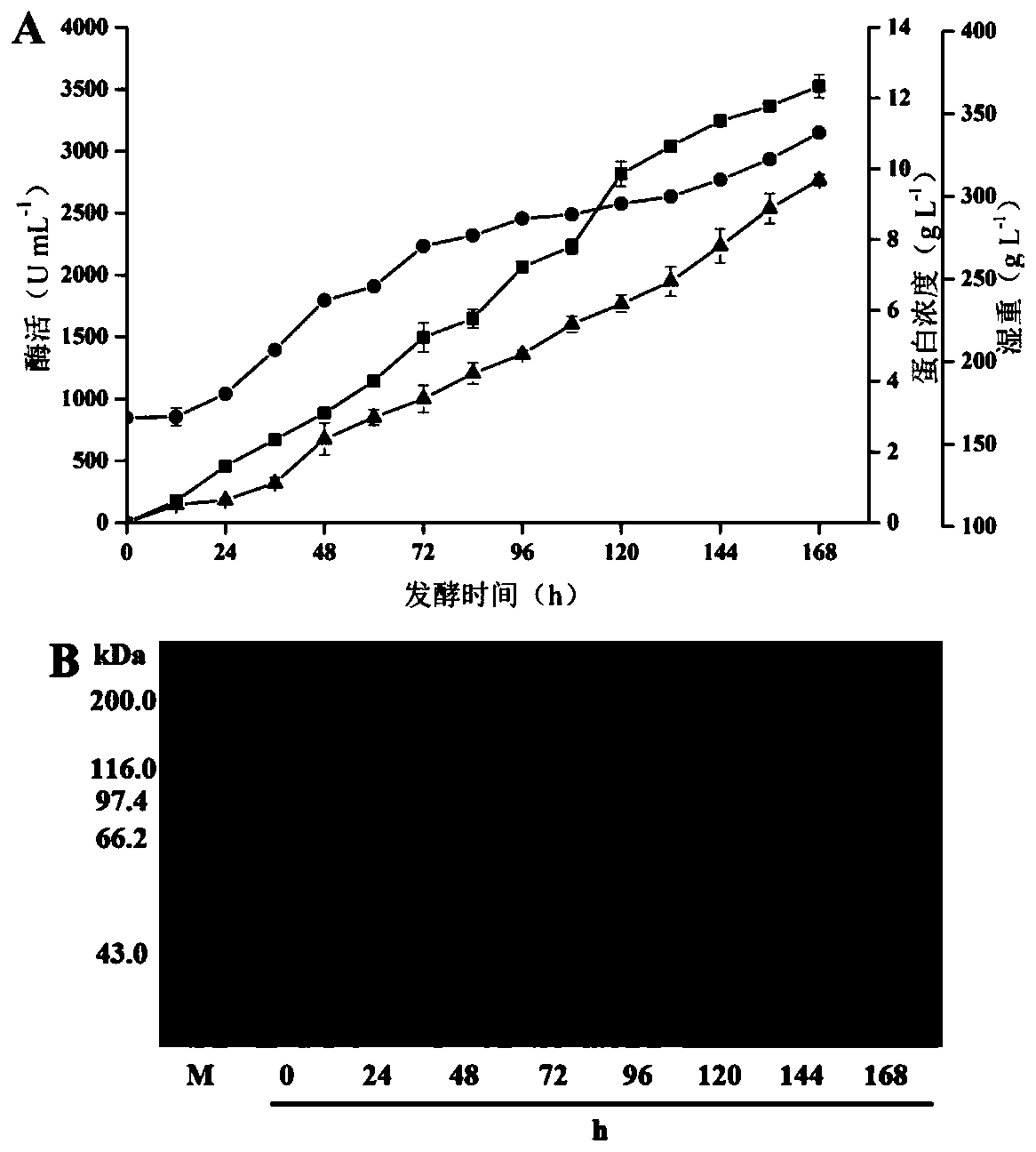
[0092] MD plate (1.34%YNB, 4 × 10 -5 % biotin, 2% glucose), His obtained from MD plate + After the transformants were scraped with sterilized water, 100 μL was spread on YPD-G418 plates with different concentrations (1% yeast extract, 2% tryptone, 2% glucose, G418 concentrations were 2, 3, 4, 6 mg / mL ). After culturing at 30°C for 3-5 days, pick the transformant and culture it in a BMGY medium shake flask for 16-18 hours, centrifuge at 3000rpm for 5 minutes, collect the bacteria, and resuspend the bacteria in BMGY medium to OD 600 is about 1.0, add glycerol in batches to express the target protein. The above culture conditions are 100mL Erlenmeyer flask with 20mL culture medium, temperature 30°C, rotation speed 200rpm. Glycerol was added every 24 hours to a final concentration of 1% for 3 days, the enzyme activity of the f...
Embodiment 3
[0133] Embodiment 3, the optimization of synthetic LNT2 condition
[0134] The amount of synthesized LNT2 was determined by HPLC. The HPLC determination conditions are: Waters XBridge BEH Amide 5 μm chromatographic column (250×4.6mm), 72% acetonitrile, column temperature 45°C, flow rate 0.5mL / min, 45min, differential (RID) detector.
[0135] Transglycoside product yield (%) = concentration of synthetic product (mM) / initial concentration of glycoside donor (mM) × 100
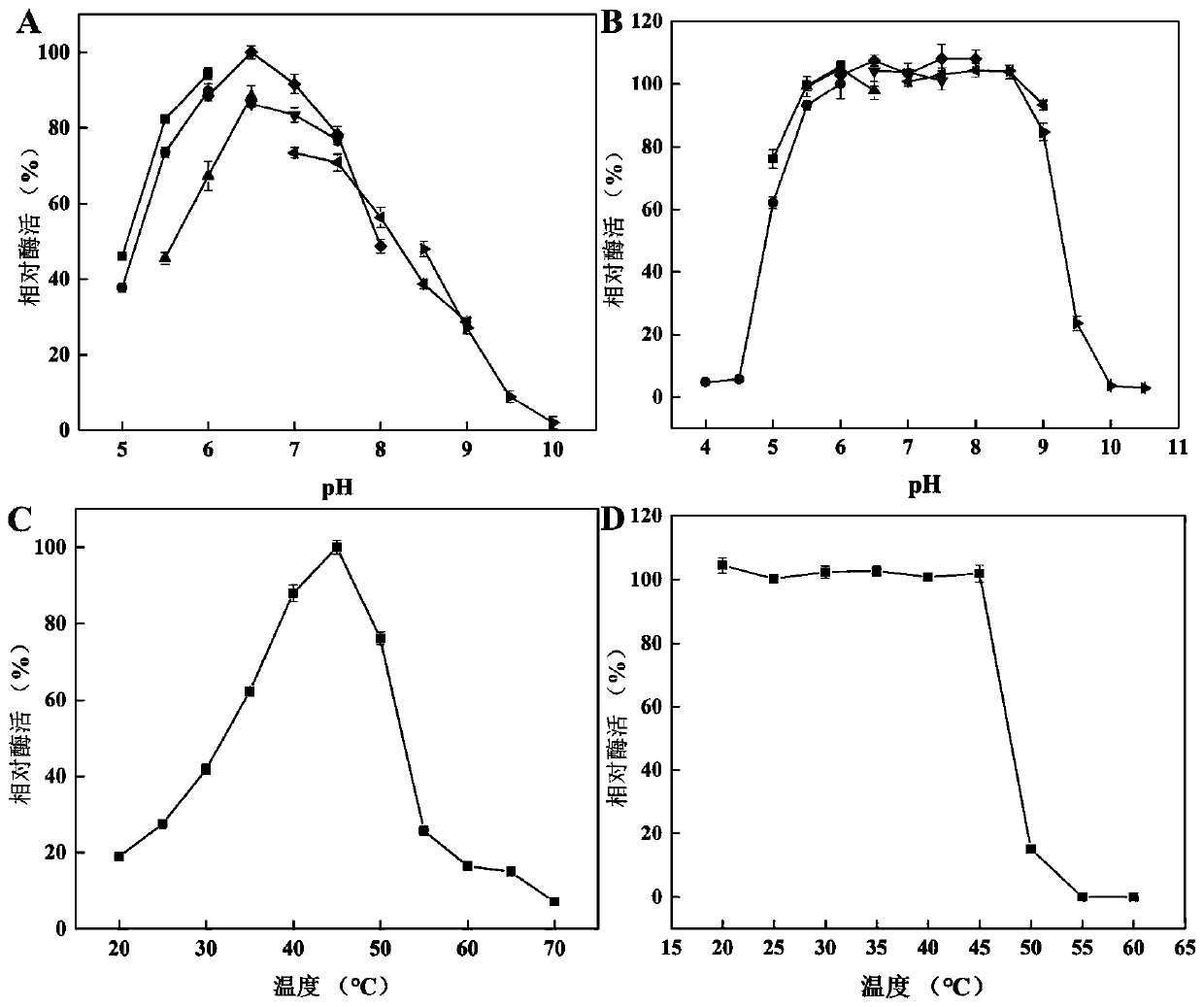
[0136] 1. Determination of optimum pH
[0137] The HaHex74 pure enzyme solution prepared in Example 2 was used as the enzyme solution to be tested, which were respectively placed in different buffer systems to determine the LNT2 content. The various buffers are as follows:
[0138] 1) MES buffer (pH 5.5-6.5)
[0139] 2) Phosphate buffer (pH 6.0-8.0)
[0140] 3) Tris-HCl buffer solution (pH 7.0-9.0)
[0141] see results Figure 4 Middle A: The optimum pH for the synthesis of LNT2 by HaHex74 is 7.5.
[01...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com