Polypeptide vaccine, preparation method and application
A polypeptide vaccine and bottle cap technology, applied in the field of vaccines, can solve the problems of difficulty in dissolving polypeptides with a single solvent and different solubility, and achieve the effects of avoiding repeated freezing and thawing, avoiding pollution, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
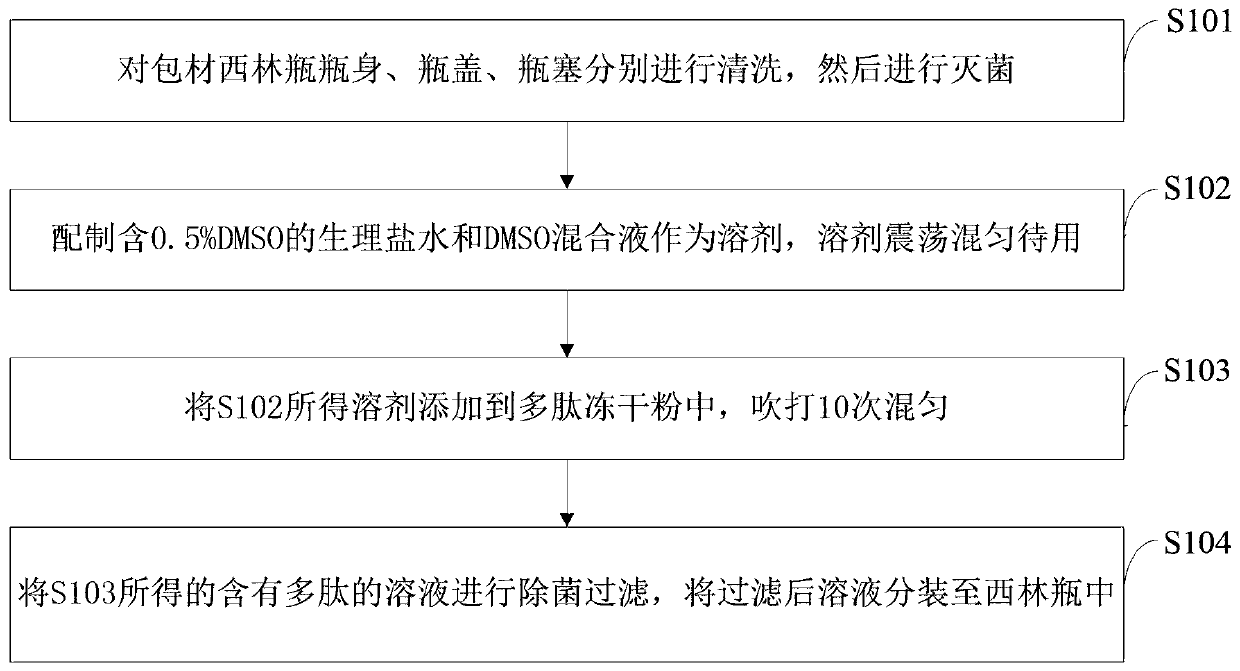
[0041] Such as figure 1 As shown, the preparation method of the polypeptide vaccine provided by the embodiments of the present invention comprises the following steps:
[0042] S101: Clean and sterilize the body, cap and stopper of the vial separately; the details are as follows: add 1 L and 500 ml of ultrapure water to 100 2ml borosilicate vials and 100 caps and stoppers, respectively, and rinse. Rinse repeatedly 10 times. The rinsed bottle body, cap and cork are placed upside down in a clean aluminum box and dried at 80°C. Put the bottle body in an aluminum box, seal the two layers with tin foil, and perform dry heat sterilization at 200°C for 3 hours. The bottle cap and bottle stopper are placed in an aluminum box, packed with kraft paper and cotton thread, and sterilized at 121°C for 20 minutes under high temperature and high pressure. After the bottle cap and the bottle stopper are sterilized, they are dried in an oven at 80°C and are ready for use.
[0043] S102: Pre...
Embodiment 1
[0048]Add 1L and 500ml ultrapure water to 100 2ml medium borosilicate vials and 100 bottle caps and stoppers, respectively, and rinse for 10 times. The rinsed bottle body, cap and cork are placed upside down in a clean aluminum box and dried at 80°C. Put the bottle body in an aluminum box, seal the two layers with tin foil, and perform dry heat sterilization at 200°C for 3 hours. The bottle cap and bottle stopper are placed in an aluminum box, packed with kraft paper and cotton thread, and sterilized at 121°C for 20 minutes under high temperature and high pressure. After the bottle cap and the bottle stopper are sterilized, they are dried in an oven at 80°C and are ready for use.
[0049] A mixture of physiological saline and DMSO containing 0.5% DMSO was prepared as a solvent, and the solvent was shaken and mixed until ready for use.
[0050] Centrifuge the synthesized lyophilized powder at 1000rpm for 5min, add 667ul solvent to 2mg polypeptide lyophilized powder, pipette a...
Embodiment 2
[0053] The personalized polypeptide vaccine prepared in Example 1 was subjected to bacterial and fungal smear tests, endotoxin and fungal D-glucan tests, and abnormal toxicity tests in Balb / c mice.
[0054] The present invention has been tested by bacteria and fungi smears, and the results show that fungi inspection: no fungi were found, and pathogenic microorganism microscopic examination: no bacteria were found;
[0055] The present invention is tested for endotoxin and fungal D-glucan, and the results show that the endotoxin is 0.101eu / ml, and the fungal D-glucan is 30.142pg / ml.
[0056] The present invention has been tested for abnormal toxicity in Balb / c mice. The detection method is: 0.5ml of polypeptide vaccine is injected into the tail vein of mice; the results show that during the abnormal toxicity test, all animal-general behaviors, appearance signs, eating and drinking activities, etc. are normal; All animals were healthy and alive.
[0057] The preparation method ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com