A kind of microsphere preparation method that improves hydrophilic drug encapsulation efficiency
A technology of hydrophilic drugs and encapsulation efficiency, which is applied in the directions of drug delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc., can solve the problems of incomplete solid-liquid separation, sacrificing yield, and uneven shape, etc. Achieve the effect of saving R&D and production costs, improving competitiveness, and reducing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
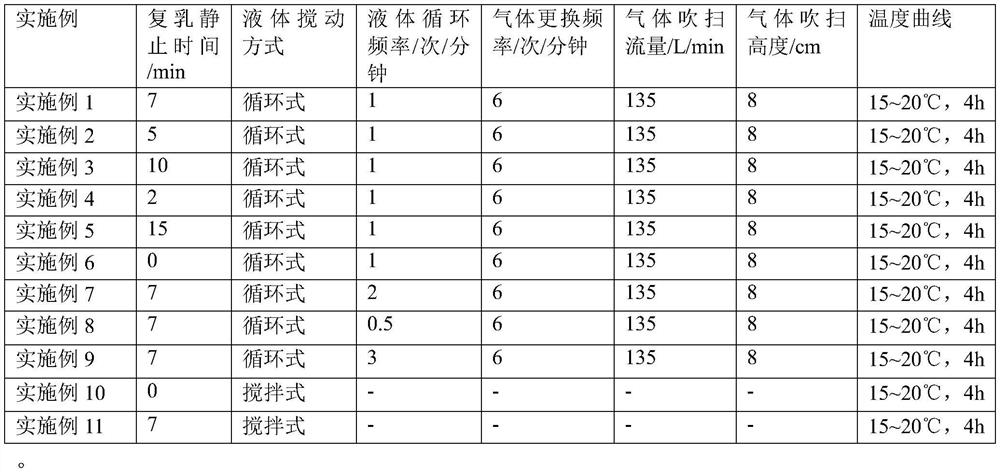
Examples
Embodiment 1
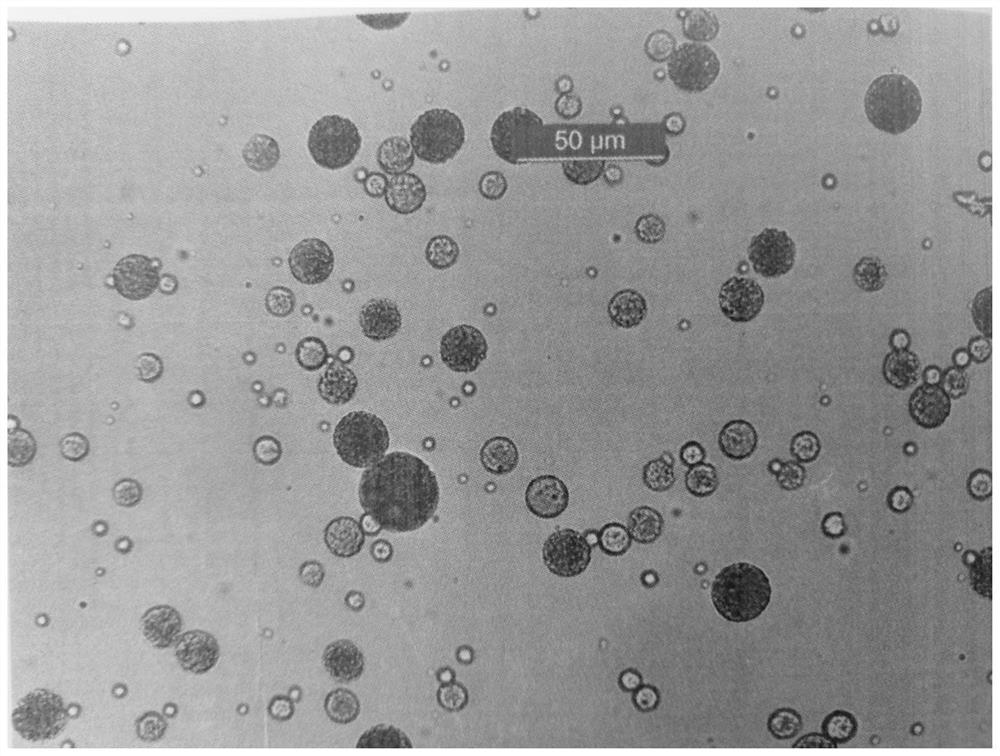
[0054] 0.78 g of leuprolide acetate was weighed and dissolved in 1.0 g of distilled water, and the solution was shaken and dissolved under heating at 55° C. to obtain an inner aqueous phase solution. 6.0 g of polylactic acid glycolic acid copolymer was weighed and dissolved in 10.0 g of dichloromethane, and dissolved by vortexing to obtain an oil phase solution. The above-mentioned inner aqueous phase solution was added to the oil phase solution, emulsified by a homogenizer (IKA, T25) to obtain a colostrum, and then rapidly cooled to 15° C. to increase the viscosity of the colostrum.
[0055] An aqueous solution (outer water phase) containing 0.25% (w / w) polyvinyl alcohol was prepared in advance, and the temperature was kept at 15° C. The above-mentioned primary emulsion and outer water kept at the same temperature were passed through an online shearing machine (IKA, Magic Lab) to uniformly disperse the primary emulsion to obtain a double emulsion with the target particle size...
Embodiment 2
[0061] 0.78 g of leuprolide acetate was weighed and dissolved in 1.0 g of distilled water, and the solution was shaken and dissolved under heating at 55° C. to obtain an inner aqueous phase solution. 6.0 g of polylactic acid glycolic acid copolymer was weighed and dissolved in 10.0 g of dichloromethane, and dissolved by vortexing to obtain an oil phase solution. The above-mentioned inner aqueous phase solution was added to the oil phase solution, emulsified by a homogenizer (IKA, T25) to obtain a colostrum, and then rapidly cooled to 15° C. to increase the viscosity of the colostrum.
[0062] An aqueous solution (outer water phase) containing 0.25% (w / w) polyvinyl alcohol was prepared in advance, and the temperature was kept at 15° C. The above-mentioned primary emulsion and outer water kept at the same temperature were passed through an online shearing machine (IKA, Magic Lab) to uniformly disperse the primary emulsion to obtain a double emulsion with the target particle size...
Embodiment 3
[0068] 0.78 g of leuprolide acetate was weighed and dissolved in 1.0 g of distilled water, and the solution was shaken and dissolved under heating at 55° C. to obtain an inner aqueous phase solution. 6.0 g of polylactic acid glycolic acid copolymer was weighed and dissolved in 10.0 g of dichloromethane, and dissolved by vortexing to obtain an oil phase solution. The above-mentioned inner aqueous phase solution was added to the oil phase solution, emulsified by a homogenizer (IKA, T25) to obtain a colostrum, and then rapidly cooled to 15° C. to increase the viscosity of the colostrum.
[0069] An aqueous solution (outer water phase) containing 0.25% (w / w) polyvinyl alcohol was pre-prepared, and the temperature was kept at 15°C, and the above-mentioned primary emulsion and outer water kept at the same temperature were passed through an online shearing machine (IKA, Magic Lab) to uniformly disperse the primary emulsion to obtain a double emulsion with the target particle size.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com