Porcine rotavirus vaccine, antigen for preparing vaccine and coding sequence of antigen
A porcine rotavirus and antigen technology, applied in the field of immunology, can solve the problems of ineffective prevention of rotavirus, and achieve the effects of low production cost, good immune effect and strong antigen specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The recombination of the antigen of embodiment 1 porcine rotavirus vaccine
[0026] In the present invention, by analyzing the VP6 gene of group A porcine rotavirus, the specific sequence fragments of highly hydrophilic regions, signal peptides and highly conserved regions are selected and combined to obtain a recombinant antigen with better immune effect, which completely contains The above-mentioned specific sequences ensure the high efficiency and specificity of the antigen to the greatest extent. The amino acid sequence of the antigen is shown in SEQ ID NO:1, and the nucleotide sequence encoding the antigen is shown in SEQ ID NO:2.
[0027] In order to increase soluble expression, SEQ ID NO:2 has been modified, and the sequence of the modified nucleotide fragment is shown in SEQ ID NO:3, that is, the cysteine at the 93rd and 227th positions of SEQ ID NO:2 Acid codons were replaced with glycine codons.
[0028] The present invention also provides a method for pre...
Embodiment 2
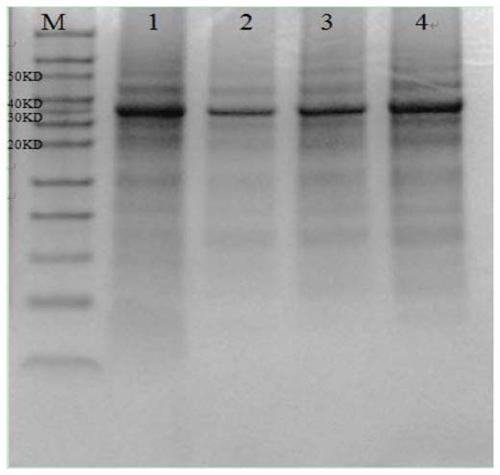
[0034] The expression of embodiment 2 antigen
[0035] 1. Cloning of target gene and construction of recombinant expression plasmid
[0036] First, artificially synthesize nucleotide fragments of SEQ ID NO:6 and SEQ ID NO:7, add 10 μl of plasmid DNA containing artificially synthesized nucleotide fragments to E.coli Top10, mix in ice bath for 30 minutes, and then heat shock at 42°C for 90s , and then immediately ice-bathed for 2 to 3 minutes. Then spread evenly on LB plates containing ampicillin (100 μg / ml), and culture overnight at 37°C. Pick the positive colony and shake the bacteria, extract the plasmid, and identify it by PCR and double enzyme digestion (NcoI / XhoI). The correct recombinant plasmid is sent to Sangon Bioengineering (Shanghai) Co., Ltd. for sequencing. The recombinant cloning plasmid and the expression vector pGEX-4T-1 were digested with NcoI and XhoI respectively, the gene fragment and the pGEX-4T-1 vector fragment were recovered, ligated overnight at 16°C ...
Embodiment 3A
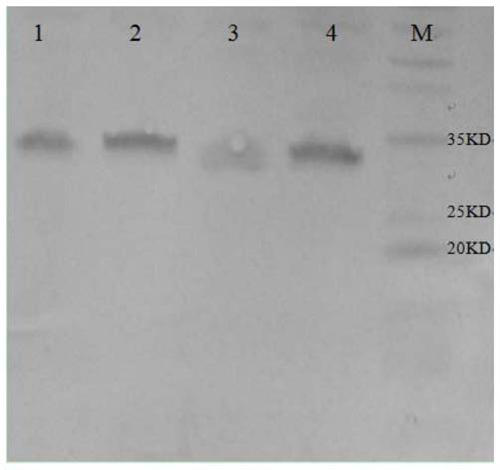
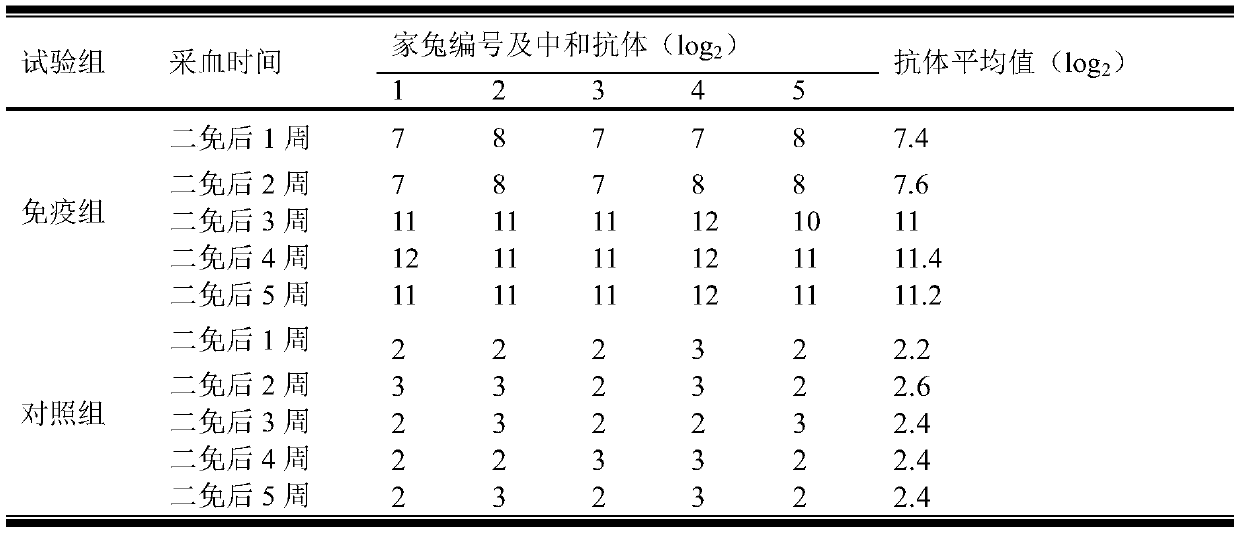
[0041] Example 3 Purification, renaturation and subunit vaccine preparation of group A porcine rotavirus VP6 structural protein
[0042] Follow the steps below for purification and renaturation: (1) Take out the induced expression bacterial solution and put it in a 50ml centrifuge tube, centrifuge at 5000r / min for 10min at 4°C, and take the supernatant; (2) Add ammonium sulfate to the supernatant to 20% saturation, after fully mixing, place at 2-8°C, 12-16 hours, centrifuge at 7000r / min for 30 minutes, discard the precipitate, and collect the supernatant; (3) add ammonium sulfate to the supernatant to 50% saturation, fully After mixing, place at 2-8°C for 12-16 hours, centrifuge at 7000r / min for 30 minutes, discard the supernatant, and collect the precipitate; (4) After weighing the precipitate, add sterilized physiological saline to redissolve at a ratio of 1:10, 7000r / min Centrifuge for 30 minutes, discard the precipitate, and collect the supernatant; (5) Add ammonium sulfat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com