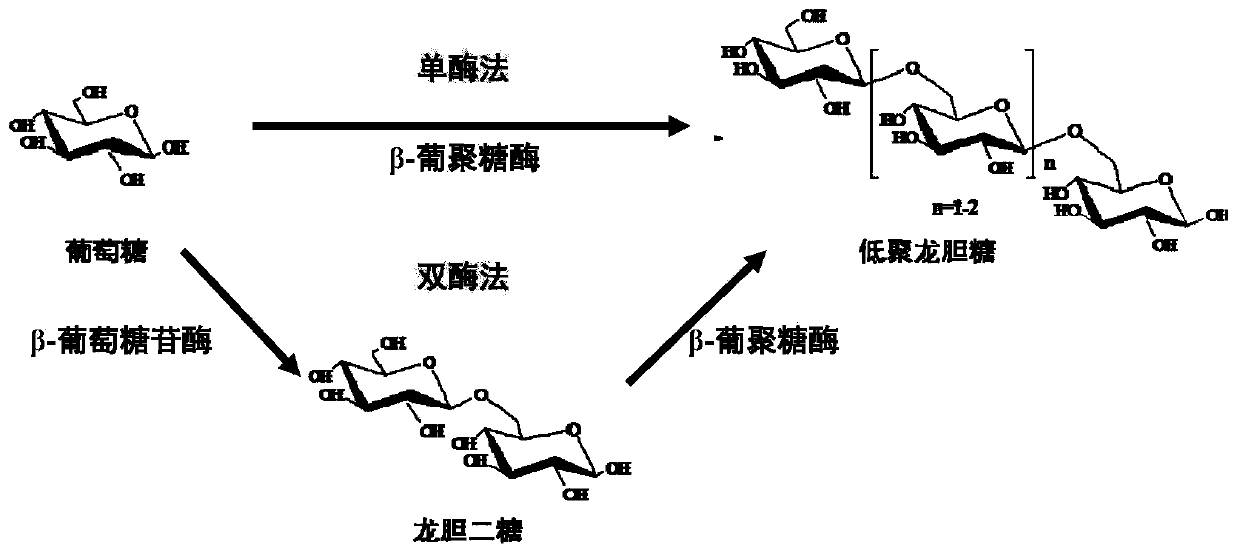
Method for preparing gentiooligosaccharide by using beta-1,6-glucanase and application of method
A technology for oligogentisose and glucanase, which is applied in the field of preparing gentiosaccharides, and can solve the problem of undetected gentiotrisaccharide oligosaccharide gentisose components and low yield of gentiosaccharide oligosaccharides. , the problem of high cost of using enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Construction of β-1,6 glucanase and β-glucosidase Pichia genetically engineered bacteria
[0052] (1) Construction of β-1,6 glucanase Pichia pastoris genetically engineered bacteria
[0053] The gene encoding β-1,6-glucanase TcBgn (nucleotide sequence shown in SEQ ID NO.3) was chemically synthesized and connected to the expression vector pPIC9K of Pichia pastoris to obtain the recombinant plasmid pPIC9K-TcBgn and transform it into Escherichia coli JM109 In the middle, after enzyme digestion verification, the recombinant plasmid pPIC9K-TcBgn was integrated into Pichia pastoris KM71 by electroporation, and then the transformation solution was spread on the MD plate, and a single clone was grown on the MD plate, and then 96 transformants were picked and transferred to Transformants with higher enzyme activity were screened on a new MD plate with a small tube of 10 mL size, which was the obtained β-1,6 glucanase Pichia genetically engineered bacteria.
[0054] P...
Embodiment 2
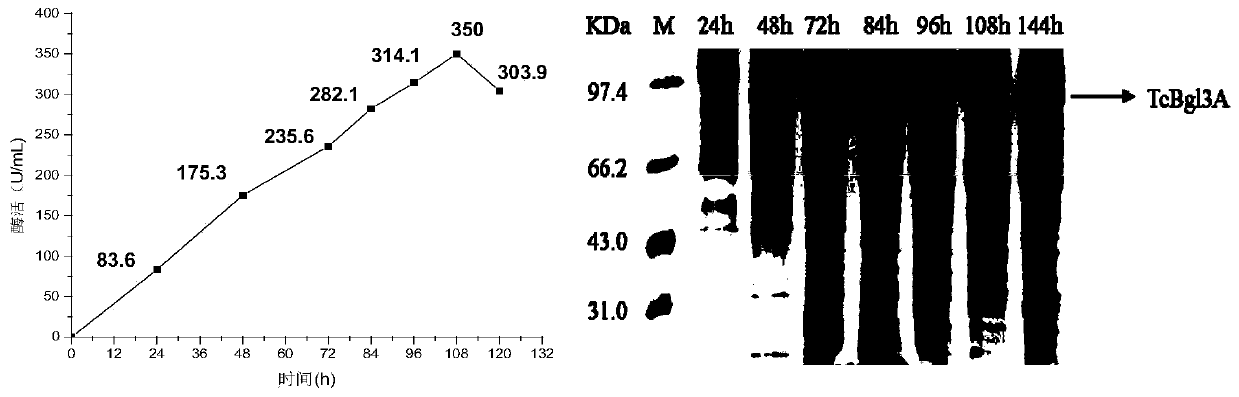
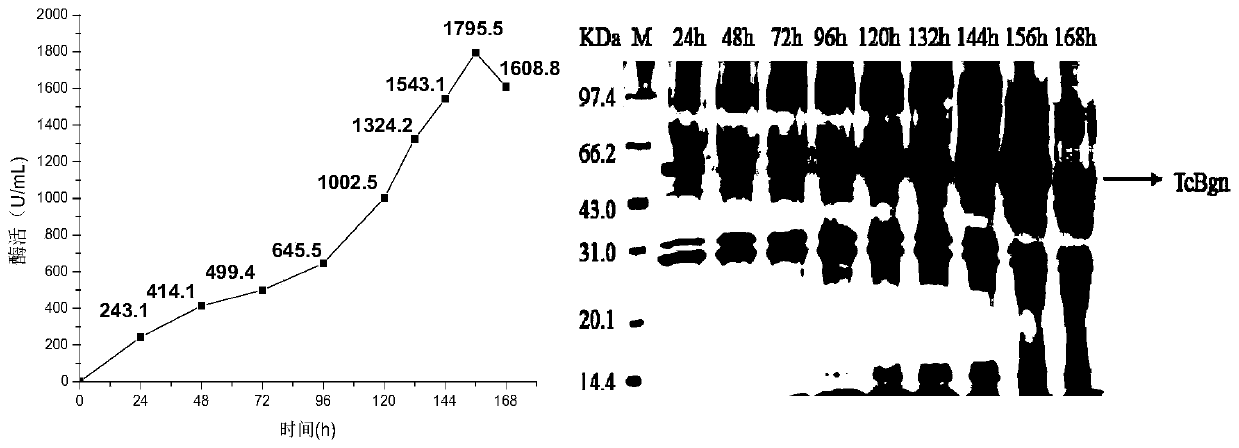
[0059] Example 2: Fermentation production of β-glucosidase and β-1,6-glucanase in a 3.6L tank
[0060] (1) Inoculate the β-1,6 glucanase Pichia genetically engineered bacteria and the β-glucosidase Pichia pastoris genetically engineered bacteria prepared in Example 1 into YPD medium respectively, at 30°C, 200rpm Cultivate for 24 hours until the OD of the seed solution 600 It is 1.3-1.5.
[0061] (2) Batch fermentation stage: inoculate the seed solution in the fermenter with 8%-12% inoculation amount, control the temperature at 28-30°C, the initial rotation speed at 180-220rpm, the initial ventilation rate at 7L / min, and the dissolved oxygen at 28-32 %, pH 4.5-5.5;
[0062] (3) Feed-fed fermentation stage: when dissolved oxygen rises to 80-100%, feed-fed culture is carried out by adding glycerin at a constant rate, controlling temperature at 28-30°C, dissolved oxygen at 28-32%, and pH 4.5-5.5;
[0063] (4) Induction culture stage: when the bacterial cell concentration OD 60...
Embodiment 3
[0076] Example 3: Enzymatic properties of β-glucosidase
[0077] The obtained β-glucosidase enzyme liquid is characterized enzymatically by the above-mentioned enzyme activity assay method, and the enzyme activity is measured at different temperatures with pNPG as a substrate, and the results show that the optimum temperature of β-glucosidase is It is 60 DEG C; Then under optimum temperature conditions, different pH gradients are set to measure the enzymatic activity of β-glucosidase, and the optimum pH of this enzyme is 4.5( Figure 4 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com