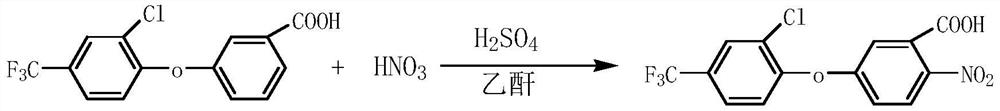
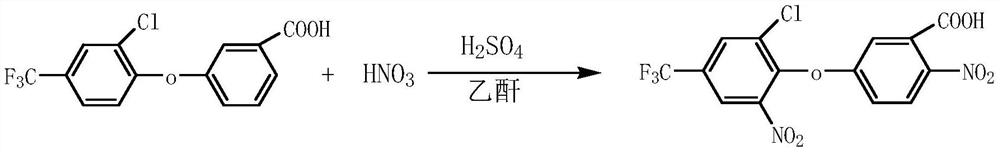
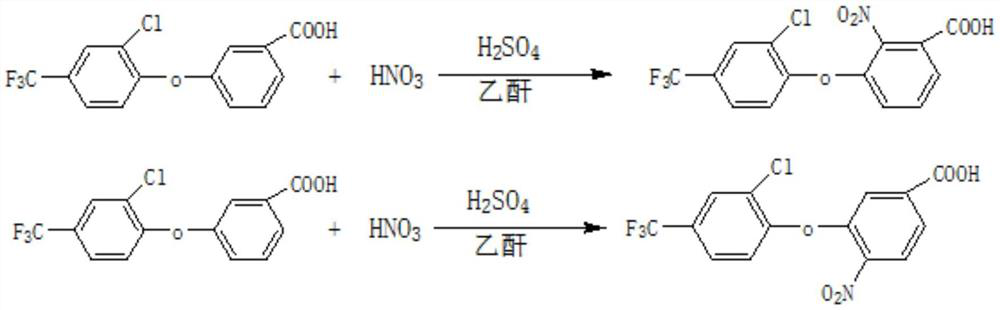
Preparation method of fomesafenbulk drug intermediate acifluorfen
A technology of fomesafen and acifluorfen is applied in the field of preparation of acifluorfen technical intermediate of fomesafen, and can solve the problems of high nitration reaction temperature, low content yield and high reaction temperature , to achieve the effect of reducing nitrification temperature, high safety and increasing content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 31.6g (actual dosage) of 3-[2-chloro-4-(trifluoromethyl)phenoxy]benzoic acid of 90.6% content and put it into a 500ml reaction bottle, add 170ml dichloroethane, stir and heat up Dissolve, cool down to about 55°C and add 30.6g of acetic anhydride, continue to cool down to 0°C, start to add 28.3g of mixed acid (0.16mol of concentrated nitric acid and 0.18mol of concentrated sulfuric acid) dropwise, the temperature does not exceed 5°C during the dropwise addition. After the dropwise addition was completed, the temperature was naturally raised to between 20°C and 25°C, and the temperature was maintained for 1.5 hours to carry out the reaction. After the reaction is completed, add 10ml of water dropwise at a controlled temperature of 50-55°C. After the dropwise addition is completed, stir at a controlled temperature of 55-60°C for 30 minutes, let stand for stratification, and remove the spent acid. After the waste acid is separated, add 100ml of water to control the te...
Embodiment 2
[0031] Weigh 31.6g (actual dosage) of 3-[2-chloro-4-(trifluoromethyl)phenoxy]benzoic acid of 90.6% content and put it into a 500ml reaction bottle, add 170ml dichloroethane, stir and heat up Dissolve, cool down to about 55°C and add 30.6g of acetic anhydride, continue to cool down to 5°C and start adding 28.3g of mixed acid (concentrated nitric acid 0.16mol and concentrated sulfuric acid 0.18mol) dropwise, the temperature during the dropping process does not exceed 10°C. After the dropwise addition was completed, the temperature was naturally raised to between 20°C and 25°C, and the temperature was maintained for 1.5 hours to carry out the reaction. After the reaction is completed, add 10ml of water dropwise at a controlled temperature of 50-55°C. After the dropwise addition is completed, stir at a controlled temperature of 55-60°C for 30 minutes, let stand for stratification, and remove the spent acid. After the waste acid is separated, add 100ml of water to control the tempe...
Embodiment 3
[0033] Weigh 31.6g (actual dosage) of 3-[2-chloro-4-(trifluoromethyl)phenoxy]benzoic acid of 90.6% content and put it into a 500ml reaction bottle, add 170ml dichloroethane, stir and heat up Dissolve, cool down to about 55°C and add 27.8g of acetic anhydride, continue to cool down to 0°C, start to add 28.3g of mixed acid (0.16mol of concentrated nitric acid and 0.18mol of concentrated sulfuric acid) dropwise, the temperature does not exceed 5°C during the dropwise addition. After the dropwise addition was completed, the temperature was naturally raised to between 20°C and 25°C, and the temperature was maintained for 1.5 hours to carry out the reaction. After the reaction is completed, add 10ml of water dropwise at a controlled temperature of 50-55°C. After the dropwise addition is completed, stir at a controlled temperature of 55-60°C for 30 minutes, let stand for stratification, and remove the spent acid. After the waste acid is separated, add 100ml of water to control the te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com