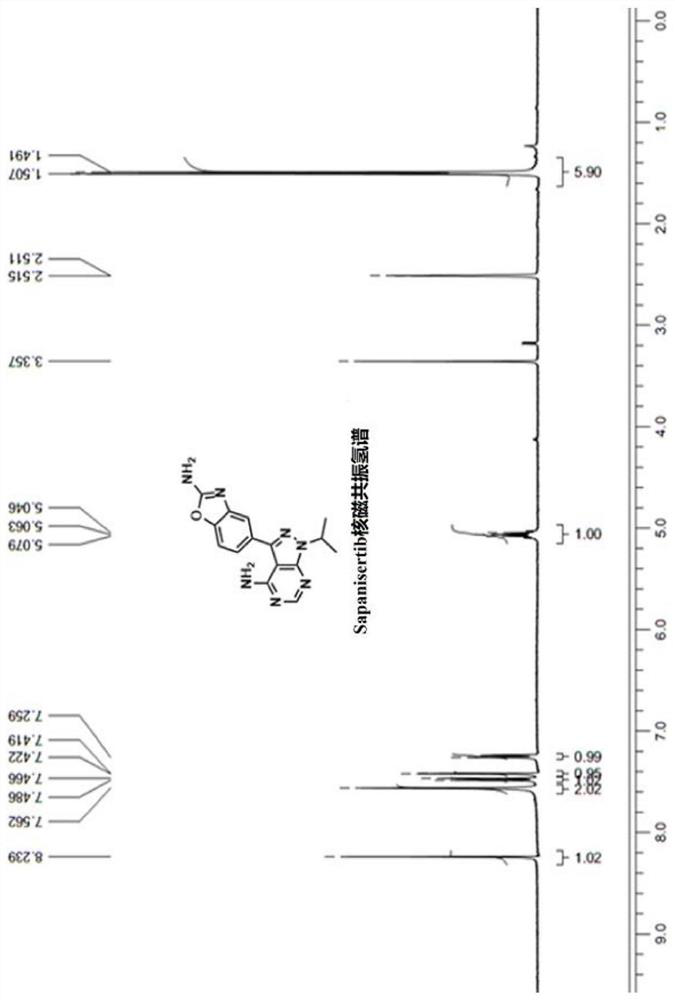
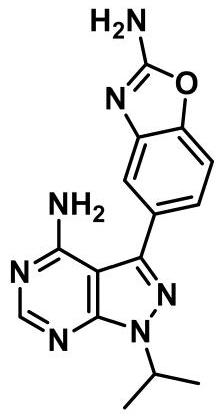
Synthesis process of anti-tumor drug Sapanisertib
An anti-tumor drug and a synthesis process technology, which is applied in the field of synthesis technology, can solve the problems of difficulty in scale-up preparation, long synthesis route and the like, and achieve the effects of good economic benefits, low production cost and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
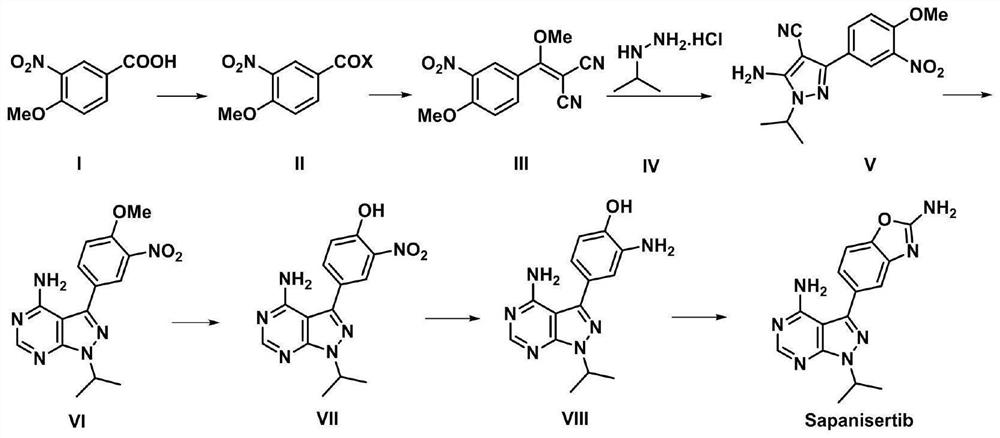
[0072] Example 1: Preparation of 4-methoxy-3-nitrobenzoyl chloride
[0073] At room temperature, 4-methoxy-3-nitrobenzoic acid (compound I) (98.6g, 0.50mols, 1.0eq), N,N-dimethylformamide (0.914g, 0.0125mols, 0.025eq) and Dichloromethane (600ml, ~6V) was added into a 1L three-necked reaction flask, and the stirring was started. Cool down in an ice-water bath, and add dropwise oxalyl chloride (95.2 g, 0.75 mols, 1.5 eq) at a controlled temperature of 20 degrees Celsius. After the addition was complete, the mixture was naturally raised to room temperature and stirred for 20 hours. The reaction solution was concentrated under reduced pressure at 45 degrees Celsius to remove dichloromethane to obtain 4-methoxy-3-nitrobenzoyl chloride as a yellow oil (108.2g, 0.502mols), with a yield of 100.4%, which was directly used in the following One step reaction.
Embodiment 2
[0074] Embodiment two: the preparation of 2-(methoxy (4-methoxy-3-nitrophenyl) methylene) malononitrile
[0075] At room temperature, 4-methoxy-3-nitrobenzoyl chloride (compound II) (108.2g, 0.50mols, 1.0eq), malononitrile (33.0g, 0.50mols, 1.0eq) and tetrahydrofuran (1100ml, ~ 10V) was added into a 2L three-necked reaction flask, and the stirring was started. Under the protection of nitrogen, the temperature was cooled in an ice-water bath, and N,N-diisopropylethylamine (193.9 g, 1.50 mols, 3.0 eq) was added dropwise at a controlled temperature of 25 degrees Celsius, and the mixture was naturally raised to room temperature and stirred for 3 hours. At room temperature, dimethyl sulfate (157.7g, 1.25mols, 2.5eq), the reaction system was heated to 75 degrees Celsius, and stirred for 22 hours.
[0076] The reaction solution was cooled to room temperature, poured into ice-water mixture (1100ml), and stirred for 30 minutes. The reaction solution was extracted with ethyl acetate (...
Embodiment 3
[0077] Example 3: Preparation of 5-amino-1-isopropyl-3-(4-methoxy-3-nitrophenyl)-1H-pyrazole-4-carbonitrile
[0078]At room temperature, 2-(methoxy(4-methoxy-3-nitrophenyl)methylene)malononitrile (compound III) (77.77g, 0.3mols, 1.0eq), isopropylhydrazine salt Add salt (compound IV) (33.18g, 0.3mols, 1.0eq) and absolute ethanol (650ml, ~8V) into a 1L three-necked reaction flask, start stirring, add triethylamine (33.4g, 0.33mols, 1.1 eq). The reaction system was heated to 60° C. and stirred for 3 hours.
[0079] The reaction solution was concentrated under reduced pressure at 50°C to 2-3 times the volume, water (400ml, ~5V) and methanol (80ml, ~1V) were added, and stirred at room temperature for 2 hours. Filter, rinse the filter cake with a methanol / water mixed solvent (1 / 2, 140ml, ~2V), and dry to obtain 5-amino-1-isopropyl-3-(4-methoxy-3-nitrophenyl )-1H-pyrazole-4-carbonitrile was a yellow solid (86.1 g, 0.286 mols), yield 95.3%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap