Preparation method of FAPGG
A compound, a furyl-based technology, applied in the field of organic synthesis for the preparation of FAPGG, can solve the problems of large environmental pollution, low total yield, high cost, etc., and achieve the effect of simple process, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
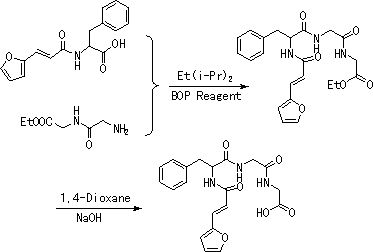
[0020] 1. Preparation of ethyl glycylidene hydrochloride. Into a 100mL three-necked flask, add 10.6g of diglyglyceride and 36.8g of absolute ethanol in sequence, cool to 0°C, slowly add 19.1g of thionyl chloride dropwise, after the addition is complete, stir and react at 40°C for 6h. The reaction mixture was rotary evaporated to remove the solvent, and then filtered to obtain a white solid, which was dried in vacuo at 40°C to obtain 15.0 g of a white solid with a yield of 94.9%. 1 HNMR (500 MHz, D 2 O), δ: 4.26~3.92(dd, J =15.0,5.0 Hz,2H),4.09~4.07(d, J =10.0 Hz,2H),3.92(s,2H),1.29~1.26(t, J =15.0,5.0 Hz,3H).
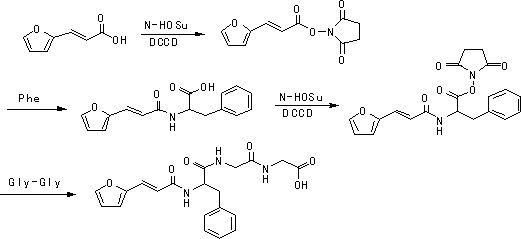
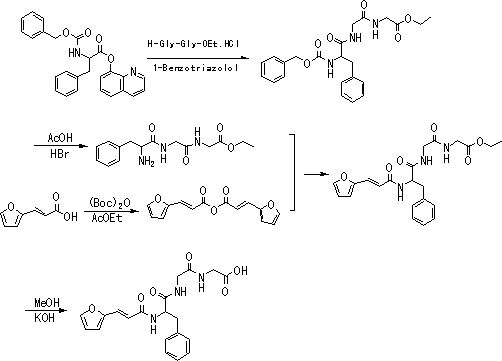
[0021] 2. Preparation of N-[3-(2-furyl)acryloyl]-L-phenylalanyl-glycyl-glycine ethyl ester. Into a 100mL three-necked flask, 5.7g N-[3-(2-furyl)acryloyl]-L-phenylalanine, 9.4g diglycidyl ethyl ester hydrochloride and 30mL of anhydrous dichloromethane were sequentially added, The mixture was cooled to 0°C, and under nitrogen, slowly added dropwise 11.9g of diisopro...
Embodiment 2
[0029] 1. The preparation of ethyl diglycopeptide hydrochloride. Into a 250mL three-neck flask, add 19.8g of diglyglyceride and 69.0g of absolute ethanol in sequence, cool to 0°C, slowly add 35.7g of thionyl chloride dropwise, after the addition is complete, stir and react at 40°C for 6h. The reaction mixture was rotary evaporated to remove the solvent, and the solid was obtained by filtration, and dried under vacuum at 40°C to obtain 28.2 g of a white solid, with a yield of 95.6%.
[0030] 2. Preparation of N-[3-(2-furyl)acryloyl]-L-phenylalanyl-glycyl-glycine ethyl ester. Add 14.3g N-[3-(2-furyl)acryloyl]-L-phenylalanine, 13.2g diglyceride ethyl ester hydrochloride and 80mL anhydrous dichloromethane to a 250mL three-necked flask successively, The mixture was cooled to 0°C, and under nitrogen, slowly added dropwise 29.7g of diisopropylethylamine (adjusted to pH 9) and BOP (44.3g) in dichloromethane solution, the dropwise addition was complete, and the reaction was The react...
Embodiment 3
[0033] 1. The preparation of ethyl diglycopeptide hydrochloride. Into a 250mL three-neck flask, add 33.0g of diglyglyceride and 115.0g of absolute ethanol in sequence, cool to 0°C, slowly add 59.5g of thionyl chloride dropwise, after the addition is complete, stir and react at 40°C for 6h. The reaction mixture was rotary evaporated to remove the solvent, and the solid was obtained by filtration, and dried under vacuum at 40°C to obtain 46.6 g of a white solid, with a yield of 94.9%.
[0034] 2. Preparation of N-[3-(2-furyl)acryloyl]-L-phenylalanyl-glycyl-glycine ethyl ester. Add 28.5g N-[3-(2-furyl)acryloyl]-L-phenylalanine, 39.3g diglyceride ethyl ester hydrochloride and 150mL anhydrous dichloromethane to a 250mL three-necked flask successively, The mixture was cooled to 0°C, and under nitrogen, slowly added dropwise 59.3g of diisopropylethylamine (adjusted to pH 9) and a solution of BOP (88.4g) in dichloromethane. After the addition was complete, the reaction was The react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com