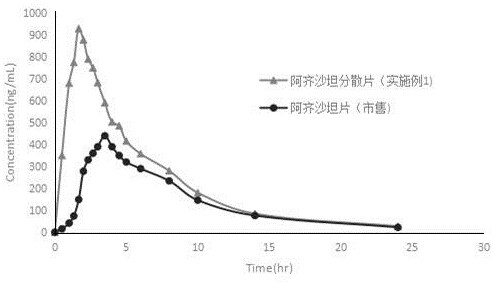
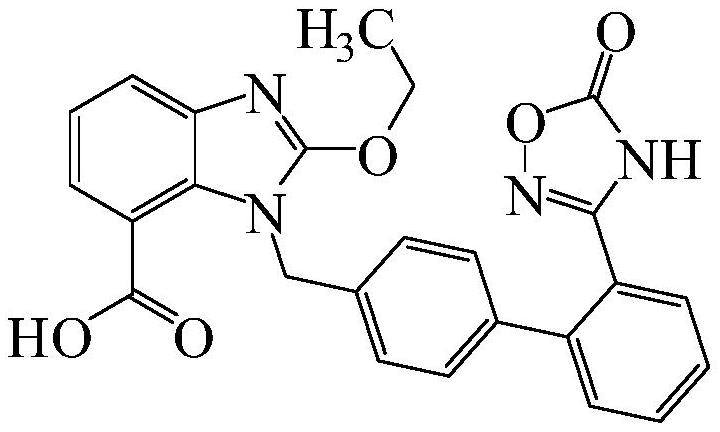
Azilsartan dispersible tablet and preparation process thereof
A technology of dispersible tablets and tablet compression, which is applied to medical preparations containing non-active ingredients, medical preparations containing active ingredients, and organic active ingredients. It can solve the problems of low dissolution and low water solubility, and achieve stable quality. The effect of uniform content and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
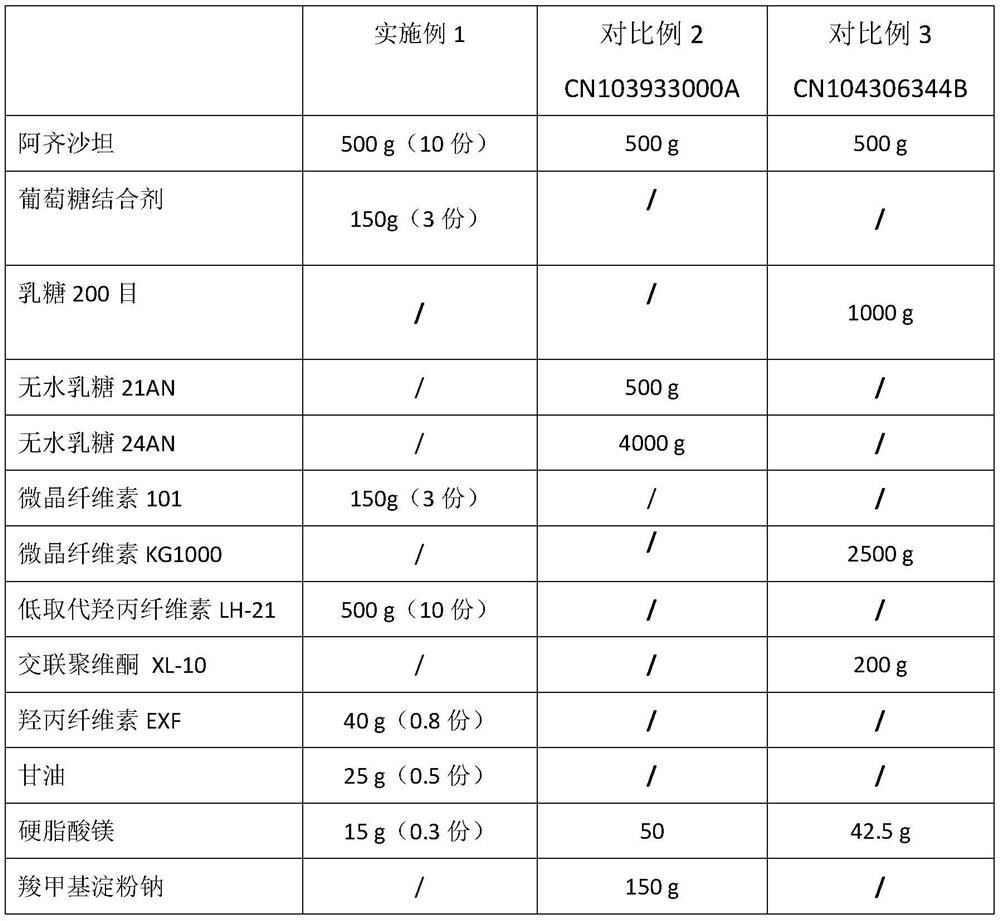
[0037] Embodiment 1, the prescription that comparative example 1-3 adopts is as shown in table 1:
[0038] Table 1
[0039]
[0040] The preparation process of embodiment 1 is as follows:
[0041]Take micronized azilsartan raw material (D90 about 25 μm), dextrose binder, low-substituted hydroxypropyl cellulose, preheat in a fluidized bed to not lower than 30 ° C, and dissolve glycerin in 3% binder Hydroxypropyl cellulose solution, the binder solution is added to the fluidized bed for granulation. During the granulation process, the air inlet temperature is controlled at 50°C, and the material temperature is greater than 27°C. After adding the binder solution, continue to dry until the weight loss on drying is ≤2.0%. Turn on the granulator to sieve with a 0.6mm screen at 500rpm, then add 200g of microcrystalline cellulose and 562.5g of low-substituted hydroxypropyl cellulose and mix for 15 minutes , and then add magnesium stearate and mix for 5 minutes. 7mm round.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com