DA beta 42, expression vector thereof, and preparation method and application of DA beta 42
An expression vector and recombinant vector technology, applied in the field of biomedicine, can solve the problems of high price and complicated operation process of Aβ42, and achieve the effects of low preparation cost and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
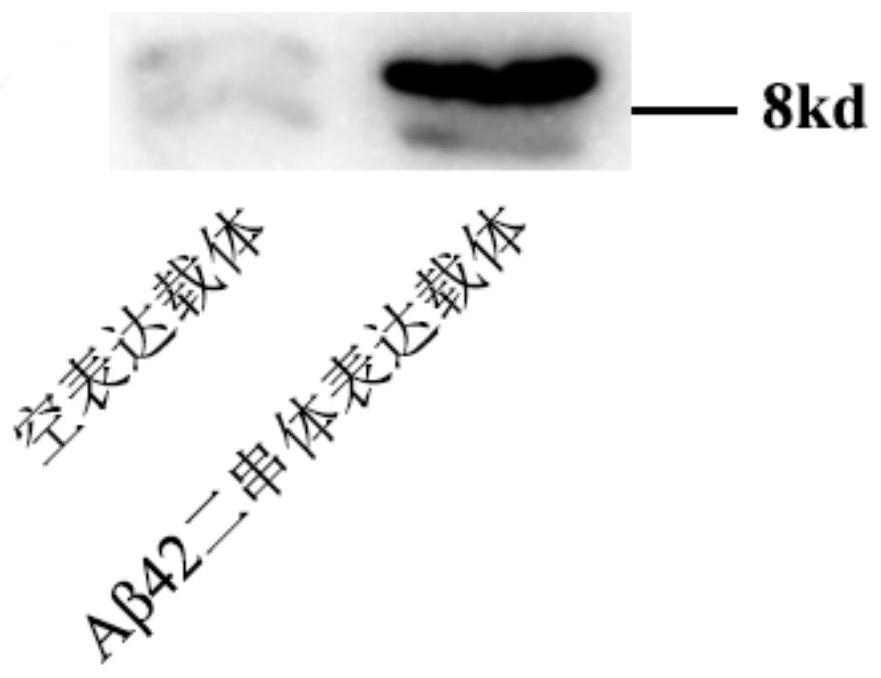
[0033] Embodiment 1, the preparation of DAβ42
[0034] Step 1. Obtain DAβ42 recombinant expression vector:
[0035] Step 1.1. Obtain the Aβ42 cDNA sequence from Genebank, as shown in SEQ ID No.2.
[0036] Step 1.2, optimize the Aβ42cDNA sequence through the prokaryotic codon system, the optimized sequence of the Aβ42cDNA prokaryotic sequence is shown in SEID No.3, and the two ends of the Aβ42cDNA sequence contain Nco I and Xho I restriction sites.
[0037] Step 1.3, cloning the optimized DAβ42 cDNA fragment obtained in step 1.2 into the pUC57 vector to obtain the pUC57-DAβ42 vector.
[0038] Step 1.4: Transform the pUC57-DAβ42 vector obtained in step 1.3 into DH5α Escherichia coli competent cells for amplification and extract the pUC57-DAβ42 vector.
[0039] Step 1.5: Digest the pUC57-DAβ42 vector obtained in step 1.4 with restriction endonucleases Nco I and Xho I respectively, and recover the DAβ42 fragment. The digestion reaction conditions are 37°C for 1h-16h, and 65°C fo...
Embodiment 2
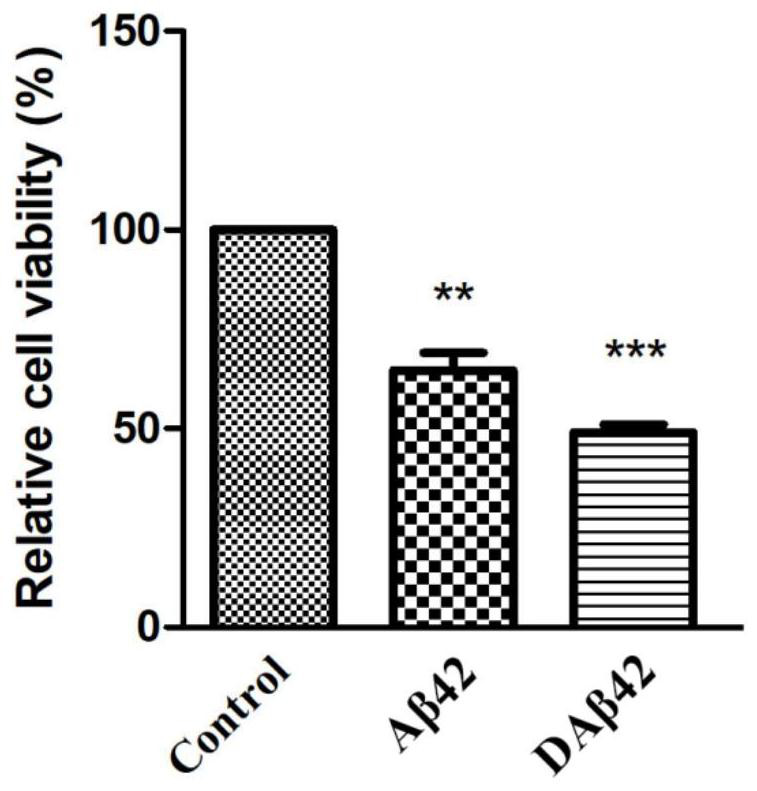
[0057] Example 2: In vitro identification of the function of DAβ42 of the present invention
[0058] 1. Culture of mouse cortical neurons
[0059] Using CO 2 Newborn mice were sacrificed by excessive inhalation, soaked in 75% ethanol for 15s to sterilize, and then the brain was taken out, placed in pre-cooled D-Hanks buffer, after peeling off the bilateral cortex under a dissecting microscope, placed in a pre-cooled 0.01M In another clean glass dish of D-Hanks solution, cut the cortex to 1mm 3 Fragments of different sizes were then digested with 0.125% trypsin at 37°C for 10-15 minutes, shaking once in the middle. After digestion, add 10% FBS to stop the digestion, blow gently with a pipette 5-8 times, let it stand for 5-8 minutes, transfer the cells together with the supernatant to a new centrifuge tube, centrifuge at 4°C, 800rpm for 5 minutes Afterwards, the supernatant was discarded, and the pellet was resuspended with DMEM+10% FBS and counted at 1.25×10 5 / cm 2 inocul...
Embodiment 3
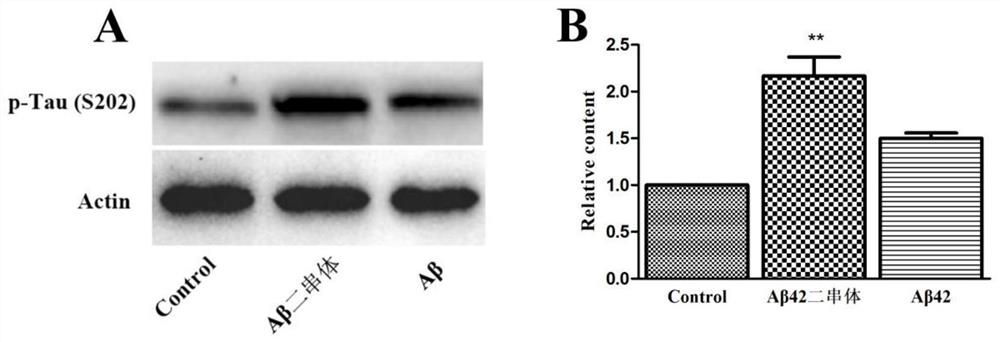
[0064] Example 3: Identification of DAβ42-induced memory impairment in mice of the present invention
[0065] Taking the artificial cerebrospinal fluid as the control group and the Aβ42 group and the DAβ42 group as the test group, the present invention injects Aβ42 and DAβ42 into the lateral ventricle of the mice. After one week, the new object recognition model is used to detect the learning and memory of the mice. The results are as follows: Figure 4 As shown, the results showed that compared with the control group, intracerebroventricular injection of Aβ42 (100 pmol) had no significant effect on the new object recognition memory of mice, while DAβ42 (100 pmol) could significantly damage the new object recognition memory of mice. It shows that DAβ42 has obvious damage ability to memory in mice, and DAβ42 has a stronger ability to damage memory than Aβ42 monomer.
[0066] In the research of the present invention, Aβ42 dyads (ie DAβ42) were prokaryotically expressed, and thes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com