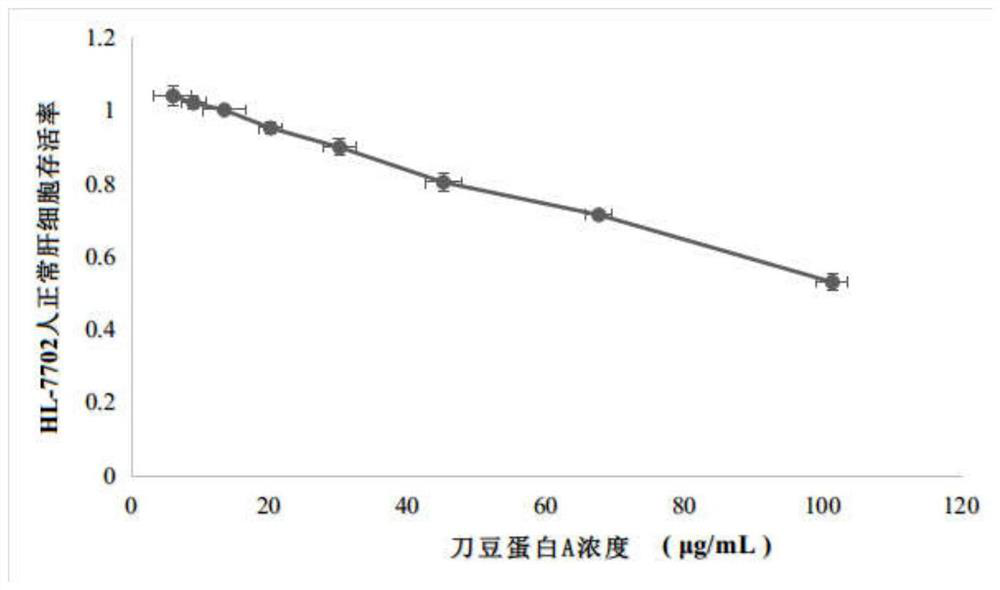
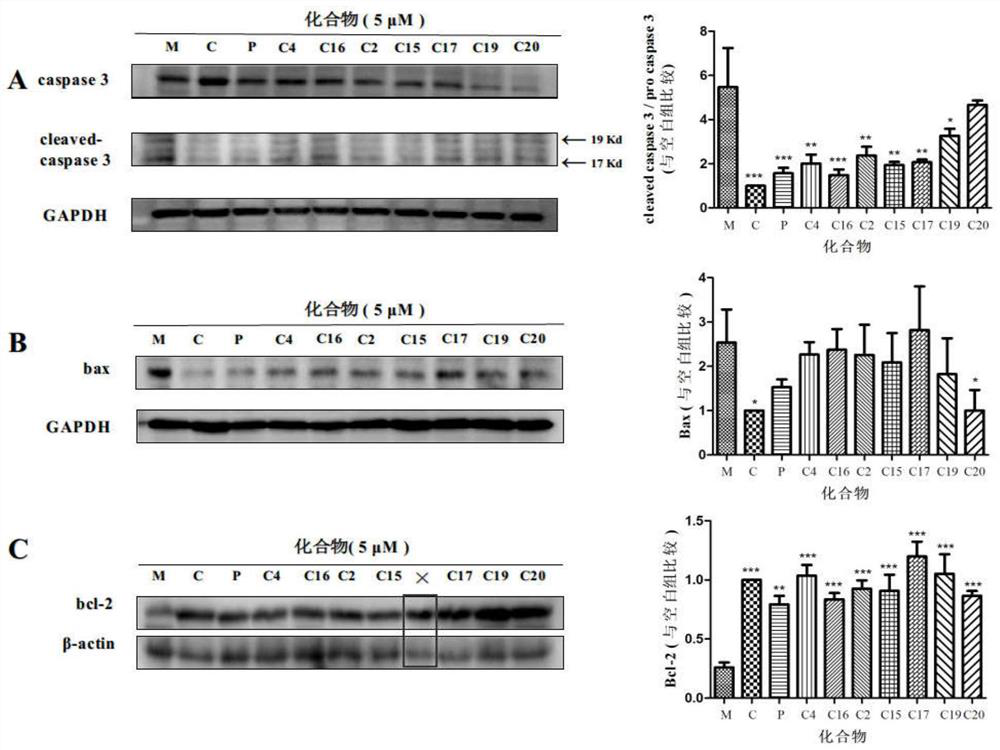
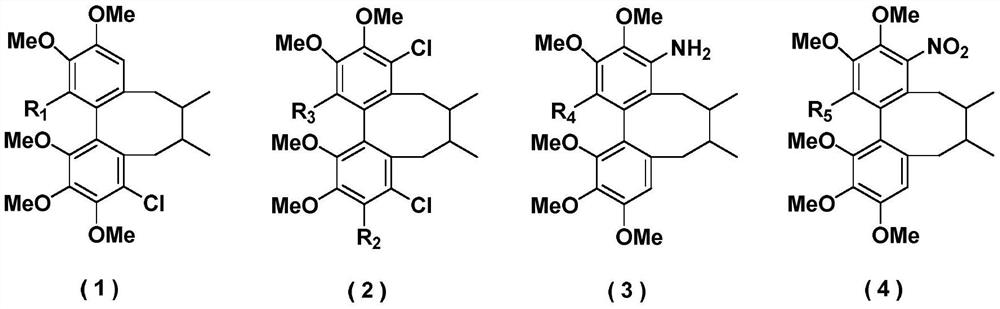
Schisanhenol derivative, preparation method and application thereof
A technology of schisandrin and its derivatives, which is applied in the field of schisandrin derivatives and its preparation, and can solve the problems of affecting drug efficacy, poor water solubility, unfavorable compound transport, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: The concrete preparation method of intermediate I-III is as follows:
[0077] Intermediate Ⅰ:
[0078]
[0079] Reaction steps: Weigh 200mg (0.50mmoL) schisandrin in a 50mL dry round bottom flask, add appropriate amount of methanol to dissolve the sample completely, then weigh 100mg (0.75mmoL) NCS in the bottle, stir and react at 20°C for 6h, wait for TLC After detecting that the reaction is complete, add water to terminate the reaction, extract 3 times with ethyl acetate, concentrate the ethyl acetate layer, and pass through silica gel column chromatography under reduced pressure (eluent is petroleum ether-ethyl acetate (V / V)=10 ︰1), and intermediate Ⅰ (197.0 mg, yield 90.8%) was prepared.
[0080] Intermediate II:
[0081]
[0082] Reaction steps: Weigh 100mg (0.25mmoL) schisandra phenol and 70mg (0.52mmoL) NCS respectively in a dry 25mL round bottom flask, then add 5mL of methanol (analytical grade) to the round bottom flask, stir and react for ...
Embodiment 2
[0087] Embodiment 2: the preparation method of schisandrin derivative C1:
[0088]
[0089] Reaction steps: Weigh 100mg of Intermediate I (0.23mmoL), 10mg of DMAP (0.08mmoL) and 59mg (0.34mmoL) of p-methoxybenzoyl chloride in a 25mL dry round bottom flask, then add 13mg of EDC·HCl (0.069 mmoL), vacuumize for 10min, under the protection of nitrogen, place in an ice-water bath, add 4mL of dry dichloromethane (DCM) under stirring, remove the ice-water bath, stir for 3h under the protection of nitrogen at room temperature, and wait for the reaction to be detected by thin-layer chromatography After completion, add water to terminate the reaction, extract 3 times with chloroform, concentrate the organic layer, and prepare C1 (121mg , the yield is 92.6%).
[0090] White solid; ESI-MS m / z: 593[M+Na] + ; 1 H-NMR (CDCl 3 , 400MHz) δ: 7.90 (2H, d, J = 8.9Hz), 6.83 (2H, d, J = 8.9Hz), 6.43 (1H, s), 3.98 (3H, s), 3.88 (3H, s), 3.82(3H,s), 3.79(3H,s), 3.70(3H,s), 3.53(3H,s), 2.37(2H...
Embodiment 3
[0091] Embodiment 3: the preparation method of schisandrin derivative C2:
[0092]
[0093] Reaction steps: Weigh 100mg of intermediate Ⅰ (0.22mmoL), 10mg of DMAP (0.08mmoL) and 57mg (0.34mmoL) of cinnamoyl chloride in a 25mL dry round bottom flask, then add 13mg of EDC·HCl (0.069mmoL), and vacuumize 10min, under nitrogen protection, placed in an ice-water bath with stirring, added 4 mL of dry dichloromethane (DCM) to remove the ice-water bath, stirred at room temperature under nitrogen protection, and stirred for 6 hours. After TLC detected that the reaction was complete, add water to terminate the reaction, chloroform Extracted 3 times, concentrated the organic layer, and prepared C2 (118 mg, yield 91.5%) by silica gel column chromatography under reduced pressure (eluent: petroleum ether-ethyl acetate (V / V)=5:1).
[0094] White solid; ESI-MS m / z: 589[M+Na] + ; 1 H-NMR (CDCl 3 , 500MHz) δ: 7.63(1H, m), 7.53(1H, m), 7.53(1H, m), 7.40(1H, m), 7.39(1H, m), 7.38(1H, m), 3.9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com